Opioidergic activation of the descending pain inhibitory system underlies placebo analgesia
- PMID: 39813331
- PMCID: PMC11734720
- DOI: 10.1126/sciadv.adp8494
Opioidergic activation of the descending pain inhibitory system underlies placebo analgesia
Abstract
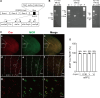
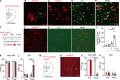
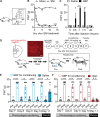
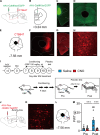
Placebo analgesia is caused by inactive treatment, implicating endogenous brain function involvement. However, the neurobiological basis remains unclear. In this study, we found that μ-opioid signals in the medial prefrontal cortex (mPFC) activate the descending pain inhibitory system to initiate placebo analgesia in neuropathic pain rats. Chemogenetic manipulation demonstrated that specific activation of μ-opioid receptor-positive (MOR+) neurons in the mPFC or suppression of the mPFC-ventrolateral periaqueductal gray (vlPAG) circuit inhibited placebo analgesia in rats. MOR+ neurons in the mPFC are monosynaptically connected and directly inhibit layer V pyramidal neurons that project to the vlPAG via GABAA receptors. Thus, intrinsic opioid signaling in the mPFC disinhibits excitatory outflow to the vlPAG by suppressing MOR+ neurons, leading to descending pain inhibitory system activation that initiates placebo analgesia. Our results shed light on the fundamental neurobiological mechanism of the placebo effect that maximizes therapeutic efficacy and reduces adverse drug effects in medical practice.
Figures


References
-
- Levine J. D., Gordon N. C., Fields H. L., The mechanism of placebo analgesia. Lancet 2, 654–657 (1978). - PubMed
-
- Petrovic P., Kalso E., Petersson K. M., Ingvar M., Placebo and opioid analgesia—Imaging a shared neuronal network. Science 295, 1737–1740 (2002). - PubMed
-
- Eippert F., Bingel U., Schoell E. D., Yacubian J., Klinger R., Lorenz J., Buchel C., Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron 63, 533–543 (2009). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

