The TRP channels serving as chemical-to-electrical signal converter
- PMID: 39813402
- PMCID: PMC12186708
- DOI: 10.1152/physrev.00012.2024
The TRP channels serving as chemical-to-electrical signal converter
Abstract
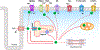
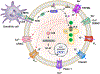
Biology uses many signaling mechanisms. Among them, calcium and membrane potential are two prominent mediators for cellular signaling. Transient receptor potential (TRP) melastatin 4 and 5 (TRPM4 and TRPM5), two calcium-activated monovalent cation-selective ion channels, offer a direct linkage between these two signals. Their activities convert a rise in the intracellular calcium level, a chemical signal, into depolarization of membrane potential, an electrical signal. Interestingly, membrane depolarization can in turn alter the electrical driving force or membrane permeability for calcium entry; hence, it offers feedback mechanisms for regulating calcium signaling. By converging two powerful cellular signals, TRPM4 and TRPM5 can contribute to many fundamental biological processes including cardiovascular biology, immunology, insulin release, chemosensation, and others. Numerous mutations in TRPM4 are linked to human hereditary cardiac and skin diseases, whereas knocking out TRPM5 in mice abolishes the perception of sweet, umami, and bitter tastes. This review summarizes what is currently known about the signaling roles of these unique TRP channels and what remains mysterious.
Keywords: calcium signaling; cardiac physiology; immune response; insulin release; membrane potential.
Figures



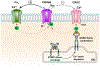
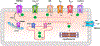

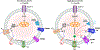
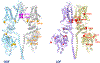
Similar articles
-
Viewpoints on the Role of Transient Receptor Potential Melastatin Channels in Cardiovascular System and Disease: A Systematic Review.Curr Probl Cardiol. 2023 Feb;48(2):101012. doi: 10.1016/j.cpcardiol.2021.101012. Epub 2021 Oct 10. Curr Probl Cardiol. 2023. PMID: 34644560
-
Blocking TRPM4 alleviates pancreatic acinar cell damage via an NMDA receptor-dependent pathway in acute pancreatitis.Theranostics. 2025 Jun 9;15(14):6901-6918. doi: 10.7150/thno.116520. eCollection 2025. Theranostics. 2025. PMID: 40585980 Free PMC article.
-
Targeting temperature-sensitive transient receptor potential channels in hypertension: far beyond the perception of hot and cold.J Hypertens. 2023 Sep 1;41(9):1351-1370. doi: 10.1097/HJH.0000000000003487. Epub 2023 Jun 9. J Hypertens. 2023. PMID: 37334542 Review.
-
Spatiotemporal calcium signaling patterns underlying opposing effects of histamine and TAS2R agonists in airway smooth muscle.Am J Physiol Lung Cell Mol Physiol. 2025 Jul 1;329(1):L70-L83. doi: 10.1152/ajplung.00058.2025. Epub 2025 May 28. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 40434402 Free PMC article.
-
Evidence-based toxicology: a comprehensive framework for causation.Hum Exp Toxicol. 2005 Apr;24(4):161-201. doi: 10.1191/0960327105ht517oa. Hum Exp Toxicol. 2005. PMID: 15957536
References
-
- Boron WF, and Boulpaep EL. Human Physiology. Elsevier, 2016.
-
- Clapham DE. Calcium signaling. Cell 131: 1047–1058, 2007. - PubMed
-
- Berridge MJ, Lipp P, and Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000. - PubMed
-
- Colquhoun D, Neher E, Reuter H, and Stevens CF. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature 294: 752–754, 1981. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

