Current developments in T-cell receptor therapy for acute myeloid leukemia
- PMID: 39813621
- PMCID: PMC12209931
- DOI: 10.1182/bloodadvances.2024014105
Current developments in T-cell receptor therapy for acute myeloid leukemia
Abstract
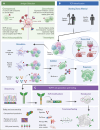
T-cell receptor (TCR) therapies are a promising modality for the treatment of cancers, with significant efforts being directed toward acute myeloid leukemia (AML), a particularly challenging disease. Chimeric antigen receptor (CAR) T cells targeting single surface antigens have shown remarkable efficacy for B-cell lymphoblastic leukemia, lymphomas, and multiple myeloma. However, AML presents formidable obstacles to the effectiveness of CAR T cells because of the widespread expression of heterogenous leukemia immunophenotypes and surface antigen targets additionally present on normal myeloid cells. TCR therapies are an evolving field of cell therapies that allow targeting intracellular antigenic peptides presented via HLA molecules. The development of TCR therapy for AML is progressing rapidly through preclinical research and successful clinical trials. This review specifically explores the antigens targeted in AML, the diverse methodologies and strategies used in TCR identification, and preclinical TCR T-cell development. The review also discusses innovative molecular designs to improve functional efficacy, mitigate safety concerns, and overcome HLA restrictions. Specific outcomes of early clinical trials targeting important antigens Wilms tumor gene 1, preferentially expressed antigen in melanoma, and minor histocompatibility antigen HA-1 are also highlighted. Ultimately, this review underscores why TCR therapy is poised to become an indispensable component of AML immunotherapy.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.B. is an inventor on a patent describing HA-1 TCR T cells that was previously licensed to Elevate Bio and has recently been licensed to Promicell Inc; received research funding from HighPass Bio, an Elevate Bio portfolio company; and has financial interests in HighPass Bio and Promicell Inc. E.B. and K.M. hold patents in adoptive cell therapy for opportunistic infection and malignancy. E.B. reports advisory board membership for IQVIA, AbbVie, MSD, Astellas, Novartis, Gilead, and Bristol Myers Squibb, and research funding from MSD. The remaining authors declare no competing financial interests.
Figures

Similar articles
-
Evolving strategies to overcome barriers in CAR-T cell therapy for acute myeloid leukemia.Expert Rev Hematol. 2024 Nov;17(11):797-818. doi: 10.1080/17474086.2024.2420614. Epub 2024 Oct 30. Expert Rev Hematol. 2024. PMID: 39439295 Review.
-
CAR-T cell therapy for treatment of acute myeloid leukemia, advances and outcomes.Mol Ther. 2025 Jun 4;33(6):2441-2453. doi: 10.1016/j.ymthe.2025.03.052. Epub 2025 Apr 2. Mol Ther. 2025. PMID: 40181544 Review.
-
Anti-CD19 CAR-T cell therapy in relapsed/refractory t(8;21) acute myeloid leukemia with aberrant CD19 expression.Front Immunol. 2025 Jul 21;16:1617589. doi: 10.3389/fimmu.2025.1617589. eCollection 2025. Front Immunol. 2025. PMID: 40761794 Free PMC article.
-
CAR-T cell therapy for cancer: current challenges and future directions.Signal Transduct Target Ther. 2025 Jul 4;10(1):210. doi: 10.1038/s41392-025-02269-w. Signal Transduct Target Ther. 2025. PMID: 40610404 Free PMC article. Review.
-
T cell receptor mimic CAR T cells targeting cathepsin G signal peptide.Leukemia. 2025 Aug;39(8):1948-1959. doi: 10.1038/s41375-025-02652-0. Epub 2025 May 28. Leukemia. 2025. PMID: 40437170
References
-
- Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87. - PubMed
-
- Creutzig U, van den Heuvel-Eibrink MM, Gibson B, et al. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120(16):3187–3205. - PubMed
-
- Sweet K, Asghari H. In: Acute Leukemias. Hematologic Malignancies. Faderl SH, Kantarjian HM, Estey E, editors. Springer International Publishing; 2021. Acute myeloid leukemia: epidemiology and etiology; pp. 3–9.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

