α-Actinin-1 deficiency in megakaryocytes causes low platelet count, platelet dysfunction, and mitochondrial impairment
- PMID: 39813624
- PMCID: PMC11925533
- DOI: 10.1182/bloodadvances.2024014805
α-Actinin-1 deficiency in megakaryocytes causes low platelet count, platelet dysfunction, and mitochondrial impairment
Abstract
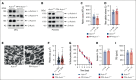
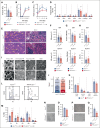
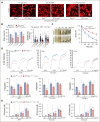
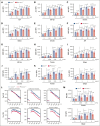
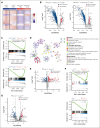
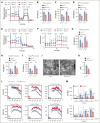
Cytoskeletal remodeling and mitochondrial bioenergetics play important roles in thrombocytopoiesis and platelet function. Recently, α-actinin-1 mutations have been reported in patients with congenital macrothrombocytopenia. However, the role and underlying mechanism of α-actinin-1 in thrombocytopoiesis and platelet function remain elusive. Using megakaryocyte (MK)-specific α-actinin-1 knockout (KO; PF4-Actn1-/-) mice, we demonstrated that PF4-Actn1-/- mice exhibited reduced platelet counts. The decreased platelet number in PF4-Actn1-/- mice was due to defects in thrombocytopoiesis. Hematoxylin and eosin staining and flow cytometry revealed a decrease in the number of MKs in the bone marrow of PF4-Actn1-/- mice. The absence of α-actinin-1 increased the proportion of 2 N-4 N MKs and decreased the proportion of 8 N-32 N MKs. Colony-forming unit-MK colony formation, the ratio of proplatelet formation-bearing MKs, and MK migration in response to stromal cell-derived factor-1 signaling were inhibited in PF4-Actn1-/- mice. Platelet spreading, clot retraction, aggregation, integrin αIIbβ3 activation, and CD62P exposure in response to various agonists were decreased in PF4-Actn1-/- platelets. Notably, PF4-Actn1-/- platelets inhibited calcium mobilization, reactive oxygen species (ROS) generation, and actin polymerization in response to collagen and thrombin. Furthermore, the PF4-Actn1-/- mice exhibited impaired hemostasis and thrombosis. Mechanistically, proteomic analysis of low-ploidy (2-4 N) and high-ploidy (≥8 N) PF4-Actn1-/- MKs revealed that α-actinin-1 deletion reduced platelet activation and mitochondrial function. PF4-Actn1-/- platelets and Actn1 KO 293T cells exhibited reduced mitochondrial membrane potential, mitochondrial ROS generation, mitochondrial calcium mobilization, and mitochondrial bioenergetics. Overall, in this study, we report that mice with α-actinin-1 deficiency in MKs exhibit low platelet count and impaired platelet function, thrombosis, and mitochondrial bioenergetics.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

