Building a Digital Health Research Platform to Enable Recruitment, Enrollment, Data Collection, and Follow-Up for a Highly Diverse Longitudinal US Cohort of 1 Million People in the All of Us Research Program: Design and Implementation Study
- PMID: 39813673
- PMCID: PMC11780292
- DOI: 10.2196/60189
Building a Digital Health Research Platform to Enable Recruitment, Enrollment, Data Collection, and Follow-Up for a Highly Diverse Longitudinal US Cohort of 1 Million People in the All of Us Research Program: Design and Implementation Study
Abstract
Background: Longitudinal cohort studies have traditionally relied on clinic-based recruitment models, which limit cohort diversity and the generalizability of research outcomes. Digital research platforms can be used to increase participant access, improve study engagement, streamline data collection, and increase data quality; however, the efficacy and sustainability of digitally enabled studies rely heavily on the design, implementation, and management of the digital platform being used.
Objective: We sought to design and build a secure, privacy-preserving, validated, participant-centric digital health research platform (DHRP) to recruit and enroll participants, collect multimodal data, and engage participants from diverse backgrounds in the National Institutes of Health's (NIH) All of Us Research Program (AOU). AOU is an ongoing national, multiyear study aimed to build a research cohort of 1 million participants that reflects the diversity of the United States, including minority, health-disparate, and other populations underrepresented in biomedical research (UBR).
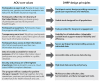
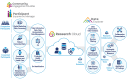
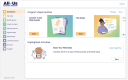
Methods: We collaborated with community members, health care provider organizations (HPOs), and NIH leadership to design, build, and validate a secure, feature-rich digital platform to facilitate multisite, hybrid, and remote study participation and multimodal data collection in AOU. Participants were recruited by in-person, print, and online digital campaigns. Participants securely accessed the DHRP via web and mobile apps, either independently or with research staff support. The participant-facing tool facilitated electronic informed consent (eConsent), multisource data collection (eg, surveys, genomic results, wearables, and electronic health records [EHRs]), and ongoing participant engagement. We also built tools for research staff to conduct remote participant support, study workflow management, participant tracking, data analytics, data harmonization, and data management.
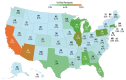
Results: We built a secure, participant-centric DHRP with engaging functionality used to recruit, engage, and collect data from 705,719 diverse participants throughout the United States. As of April 2024, 87% (n=613,976) of the participants enrolled via the platform were from UBR groups, including racial and ethnic minorities (n=282,429, 46%), rural dwelling individuals (n=49,118, 8%), those over the age of 65 years (n=190,333, 31%), and individuals with low socioeconomic status (n=122,795, 20%).
Conclusions: We built a participant-centric digital platform with tools to enable engagement with individuals from different racial, ethnic, and socioeconomic backgrounds and other UBR groups. This DHRP demonstrated successful use among diverse participants. These findings could be used as best practices for the effective use of digital platforms to build and sustain cohorts of various study designs and increase engagement with diverse populations in health research.
Keywords: biomedical research; cohort studies; database management system; decentralization; digital health technology; health disparities; longitudinal studies; minority populations; precision medicine; vulnerable populations.
©Dave Klein, Aisha Montgomery, Mark Begale, Scott Sutherland, Sherilyn Sawyer, Jacob L McCauley, Letheshia Husbands, Deepti Joshi, Alan Ashbeck, Marcy Palmer, Praduman Jain. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 15.01.2025.
Conflict of interest statement
Conflicts of Interest: MB, AM, DK, S Sutherland, DJ, AA, MP, and PJ are employees of Vibrent Health, Inc. MB, S Sawyer, JLM, DK, and PJ are affiliated with the All of Us Research Program.
Figures



References
-
- Caruana EJ, Roman M, Hernández-Sánchez J, Solli P. Longitudinal studies. J Thorac Dis. 2015 Nov;7(11):E537–E540. doi: 10.3978/j.issn.2072-1439.2015.10.63. https://europepmc.org/abstract/MED/26716051 jtd-07-11-E537 - DOI - PMC - PubMed
-
- Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. 2016 Oct 13;538(7624):161–164. doi: 10.1038/538161a. https://europepmc.org/abstract/MED/27734877 538161a - DOI - PMC - PubMed
-
- Allison K, Patel D, Kaur R. Assessing multiple factors affecting minority participation in clinical trials: development of the Clinical Trials Participation Barriers Survey. Cureus. 2022 Apr;14(4):e24424. doi: 10.7759/cureus.24424. https://europepmc.org/abstract/MED/35637812 - DOI - PMC - PubMed
-
- Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016 Oct;23(4):327–337. doi: 10.1177/107327481602300404. https://journals.sagepub.com/doi/10.1177/107327481602300404?url_ver=Z39.... - DOI - DOI - PMC - PubMed
-
- Ejiogu N, Norbeck JH, Mason MA, Cromwell BC, Zonderman AB, Evans MK. Recruitment and retention strategies for minority or poor clinical research participants: lessons from the Healthy Aging in Neighborhoods of Diversity across the Life Span study. Gerontologist. 2011 Jun;51 Suppl 1(Suppl 1):S33–S45. doi: 10.1093/geront/gnr027. https://europepmc.org/abstract/MED/21565817 gnr027 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

