Implementation of model-informed precision dosing for tamoxifen therapy in patients with breast cancer: A prospective intervention study
- PMID: 39813819
- PMCID: PMC11783121
- DOI: 10.1016/j.breast.2025.103880
Implementation of model-informed precision dosing for tamoxifen therapy in patients with breast cancer: A prospective intervention study
Abstract
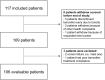
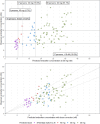
Tamoxifen is an estrogen-receptor (ER) antagonist, used as adjuvant treatment of ER-positive breast cancer. It is converted by CYP2D6 into endoxifen, its most active metabolite. Patients with endoxifen plasma concentrations <16 nM face a higher risk of recurrence. The use of a priori model-informed precision dosing (MIPD) may lead to faster target attainment and thus potentially improve patient outcomes. In total, 106 evaluable patients were prospectively included in this single-arm MIPD-intervention study. Patients received a model-predicted tamoxifen dose when starting tamoxifen-treatment (65.1 % of patients received 20 mg, 16.0 % received 30 mg and 18.9 % received 40 mg). Seventy-five percent of the 40 mg group was predicted to be unable to reach the threshold of 16 nM despite receiving the highest registered dose. After attaining steady-state, 84.0 % of patients reached endoxifen levels ≥16 nM, which was not significantly higher compared to a historical control cohort (77.9 %, p = 0.17). The model showed adequate performance and correctly identified patients requiring 40 mg tamoxifen. Endoxifen samples that were acquired 4-6 weeks after treatment initiation, are informative of steady-state endoxifen levels and can be used to inform MIPD and adjust tamoxifen dosing prior to steady-state attainment. In this first MIPD implementation study for patients treated with tamoxifen, MIPD did lead to more patients achieving endoxifen levels ≥16 nM as compared to the one-dose-fits-all strategy, albeit insignificant. This may partly be explained by a larger proportion of patients who were recommended to switch to an aromatase inhibitor (AI) in the intervention cohort. In conclusion, MIPD seems beneficial compared to one-size-fits-all-dosing, but TDM still remains an important addition.
Keywords: Breast cancer; Endoxifen; Estrogen receptor positive; Hormone therapy; Model informed precision dosing; Tamoxifen; Therapeutic drug monitoring.
Copyright © 2025 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declared no competing interests for this work.
Figures
References
-
- Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1992 Jan 4;339(8784):1–15. - PubMed
-
- Fisher B., Dignam J., Bryant J., DeCillis A., Wickerham D.L., Wolmark N., et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst. 1996 Nov 6;88(21):1529–1542. - PubMed
-
- Lim Y.C., Li L., Desta Z., Zhao Q., Rae J.M., Flockhart D.A., et al. Endoxifen, a secondary metabolite of tamoxifen, and 4-OH-tamoxifen induce similar changes in global gene expression patterns in MCF-7 breast cancer cells. J Pharmacol Exp Therapeut. 2006 Aug;318(2):503–512. - PubMed
-
- Johnson M.D., Zuo H., Lee K., Trebley J.P., Rae J.M., Weatherman R.V., et al. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85(2):151–159. - PubMed
-
- Borges S., Desta Z., Li L., Skaar T.C., Ward B.A., Nguyen A., et al. Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther. 2006;80(1):61–74. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

