ApoE3 R136S binds to Tau and blocks its propagation, suppressing neurodegeneration in mice with Alzheimer's disease
- PMID: 39814008
- PMCID: PMC12376192
- DOI: 10.1016/j.neuron.2024.12.015
ApoE3 R136S binds to Tau and blocks its propagation, suppressing neurodegeneration in mice with Alzheimer's disease
Abstract
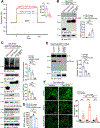
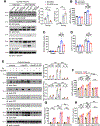
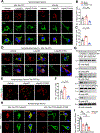
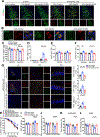
PSEN1 E280A carrier for the APOE3 Christchurch variant (R136S) is protected against Alzheimer's disease (AD) symptoms with a distinct anatomical pattern of Tau pathology. However, the molecular mechanism accounting for this protective effect remains incompletely understood. Here, we show that the ApoE3 R136S mutant strongly binds to Tau and reduces its uptake into neurons and microglia compared with ApoE3 wild type (WT), diminishing Tau fragmentation by asparagine endopeptidase (AEP), proinflammatory cytokines by Tau pre-formed fibrils (PFFs) or β-amyloid (Aβ), and neurotoxicity. Further, ApoE3 R136S demonstrates more robust effects in attenuating AEP activation and Tau PFF spreading in the brains of both 5xFAD and Tau P301S mice than in ApoE3 WT, leading to improved cognitive functions. Thus, our findings support the idea that ApoE3 R136S strongly binds Tau and decreases its cellular uptake, abrogating Tau pathology propagation in AD brains.
Keywords: AEP; ApoE3 R136S; cognitive dysfunctions; neurofibrillary tangles; pre-formed fibrils.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Haass C (1996). Presenile because of presenilin: the presenilin genes and early onset Alzheimer’s disease. Curr Opin Neurol 9, 254–259. - PubMed
-
- Arboleda-Velasquez JF, Lopera F, O’Hare M, Delgado-Tirado S, Marino C, Chmielewska N, Saez-Torres KL, Amarnani D, Schultz AP, Sperling RA, et al. (2019). Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nat Med 25, 1680–1683. 10.1038/s41591-019-0611-3. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

