The Toxoplasma rhoptry protein ROP55 is a major virulence factor that prevents lytic host cell death
- PMID: 39814722
- PMCID: PMC11736080
- DOI: 10.1038/s41467-025-56128-x
The Toxoplasma rhoptry protein ROP55 is a major virulence factor that prevents lytic host cell death
Abstract
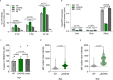
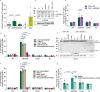
Programmed-cell death is an antimicrobial defense mechanism that promotes clearance of intracellular pathogens. Toxoplasma counteracts host immune defenses by secreting effector proteins into host cells; however, how the parasite evades lytic cell death and the effectors involved remain poorly characterized. We identified ROP55, a rhoptry protein that promotes parasite survival by preventing lytic cell death in absence of IFN-γ stimulation. RNA-Seq analysis revealed that ROP55 acts as a repressor of host pro-inflammatory responses. In THP-1 monocytes ΔROP55 infection increased NF-κB p65 nuclear translocation, IL-1β production, and GSDMD cleavage compared to wild type or complemented parasites. ΔROP55 infection also induced RIPK3-dependent necroptosis in human and mouse primary macrophages. Moreover, ΔROP55 parasites were significantly impaired in virulence in female mice and prevented NF-κB activation and parasite clearance in mBMDM. These findings place ROP55 as a major virulence factor, dampening lytic cell death and enabling Toxoplasma to evade clearance from infected cells.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures







Similar articles
-
Toxoplasma gondii parasites induce a localized myeloid cell immune response surrounding parasites in the brain during acute infection.mBio. 2025 Jul 9;16(7):e0081025. doi: 10.1128/mbio.00810-25. Epub 2025 Jun 10. mBio. 2025. PMID: 40492741 Free PMC article.
-
A cathepsin C-like protease mediates the post-translation modification of Toxoplasma gondii secretory proteins for optimal invasion and egress.mBio. 2023 Aug 31;14(4):e0017423. doi: 10.1128/mbio.00174-23. Epub 2023 Jun 16. mBio. 2023. PMID: 37326431 Free PMC article.
-
Toxoplasma gondii PPM3H regulates the parasite virulence and modulates host immune and inflammatory responses in mice.Vet Res. 2025 Aug 26;56(1):171. doi: 10.1186/s13567-025-01603-y. Vet Res. 2025. PMID: 40859392 Free PMC article.
-
Modulation of innate immunity by Toxoplasma gondii virulence effectors.Nat Rev Microbiol. 2012 Nov;10(11):766-78. doi: 10.1038/nrmicro2858. Nat Rev Microbiol. 2012. PMID: 23070557 Free PMC article. Review.
-
Toxoplasma gondii effectors are master regulators of the inflammatory response.Trends Parasitol. 2011 Nov;27(11):487-95. doi: 10.1016/j.pt.2011.08.001. Epub 2011 Sep 3. Trends Parasitol. 2011. PMID: 21893432 Free PMC article. Review.
References
-
- Pappas, G., Roussos, N. & Falagas, M. E. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol.39, 1385–1394 (2009). - PubMed
-
- Montoya, J. G. & Liesenfeld, O. Toxoplasmosis. Lancet (Lond., Engl.)363, 1965–1976 (2004). - PubMed
-
- Demar, M. et al. Fatal outbreak of human toxoplasmosis along the Maroni River: epidemiological, clinical, and parasitological aspects. Clin. Infect. Dis.: Off. Publ. Infect. Dis. Soc. Am.45, e88–e95 (2007). - PubMed
MeSH terms
Substances
Grants and funding
- NIFA-Hatch-225935/UC | University of California, Riverside (UC Riverside)
- R01 AI136511/AI/NIAID NIH HHS/United States
- R01 AI136511 and R21 AI142506/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI120846/AI/NIAID NIH HHS/United States
- R01AI120846 and R21AI156452/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

