Intranasally administrated fusion-inhibitory lipopeptides block SARS-CoV-2 infection in mice and enable long-term protective immunity
- PMID: 39814955
- PMCID: PMC11735783
- DOI: 10.1038/s42003-025-07491-4
Intranasally administrated fusion-inhibitory lipopeptides block SARS-CoV-2 infection in mice and enable long-term protective immunity
Abstract
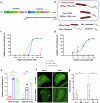
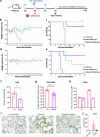
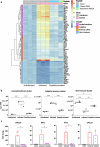
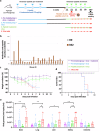
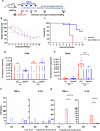
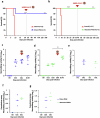
We have assessed antiviral activity and induction of protective immunity of fusion-inhibitory lipopeptides derived from the C-terminal heptad-repeat domain of SARS-CoV-2 spike glycoprotein in transgenic mice expressing human ACE2 (K18-hACE2). The lipopeptides block SARS-CoV-2 infection in cell lines and lung-derived organotypic cultures. Intranasal administration in mice allows the maintenance of homeostatic transcriptomic immune profile in lungs, prevents body-weight loss, decreases viral load and shedding, and protects mice from death caused by SARS-CoV-2 variants. Prolonged administration of high-dose lipopeptides has neither adverse effects nor impairs peptide efficacy in subsequent SARS-CoV-2 challenges. The peptide-protected mice develop cross-reactive neutralizing antibodies against both SARS-CoV-2 used for the initial infection and recently circulating variants, and are completely protected from a second lethal infection, suggesting that they developed SARS-CoV-2-specific immunity. This strategy provides an additional antiviral approach in the global effort against COVID-19 and may contribute to development of rapid responses against emerging pathogenic viruses.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.P. and A.M. anticipate future financial interest in Thylacine Biotherapeutics, a company established to develop lipopeptide antiviral therapeutics. A.M., M.P., B.H. and C.M. are inventors of several provisional patent applications related to the use of antiviral lipopeptides.
Figures
Similar articles
-
Fc-binding nanodisc restores antiviral efficacy of antibodies with reduced neutralizing effects against evolving SARS-CoV-2 variants.J Nanobiotechnology. 2025 Jan 24;23(1):44. doi: 10.1186/s12951-025-03100-y. J Nanobiotechnology. 2025. PMID: 39856746 Free PMC article.
-
MVA-based vaccine candidates expressing SARS-CoV-2 prefusion-stabilized spike proteins of the Wuhan, Beta or Omicron BA.1 variants protect transgenic K18-hACE2 mice against Omicron infection and elicit robust and broad specific humoral and cellular immune responses.Front Immunol. 2024 Aug 29;15:1420304. doi: 10.3389/fimmu.2024.1420304. eCollection 2024. Front Immunol. 2024. PMID: 39267752 Free PMC article.
-
The lethal K18-hACE2 knock-in mouse model mimicking the severe pneumonia of COVID-19 is practicable for antiviral development.Emerg Microbes Infect. 2024 Dec;13(1):2353302. doi: 10.1080/22221751.2024.2353302. Epub 2024 May 26. Emerg Microbes Infect. 2024. PMID: 38753462 Free PMC article.
-
Ronapreve (REGN-CoV; casirivimab and imdevimab) reduces the viral burden and alters the pulmonary response to the SARS-CoV-2 Delta variant (B.1.617.2) in K18-hACE2 mice using an experimental design reflective of a treatment use case.Microbiol Spectr. 2024 Aug 6;12(8):e0391623. doi: 10.1128/spectrum.03916-23. Epub 2024 Jul 16. Microbiol Spectr. 2024. PMID: 39012120 Free PMC article.
-
Discovery and development of INNA-051, a TLR2/6 agonist for the prevention of complications resulting from viral respiratory infections.Antiviral Res. 2025 Feb;234:106063. doi: 10.1016/j.antiviral.2024.106063. Epub 2024 Dec 27. Antiviral Res. 2025. PMID: 39733845 Review.
Cited by
-
Development of nebulized inhalation delivery for fusion-inhibitory lipopeptides to protect non-human primates against Nipah-Bangladesh infection.Antiviral Res. 2025 Mar;235:106095. doi: 10.1016/j.antiviral.2025.106095. Epub 2025 Jan 25. Antiviral Res. 2025. PMID: 39870114 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- RO1 AI160961/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- R01 AI160953/AI/NIAID NIH HHS/United States
- RO1 AI160953/Foundation for the National Institutes of Health (Foundation for the National Institutes of Health, Inc.)
- ANR-20-COVI-000/Agence Nationale de la Recherche (French National Research Agency)
- R01 AI160961/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

