Deep brain stimulation of the anterior cingulate cortex reduces opioid addiction in preclinical studies
- PMID: 39815019
- PMCID: PMC11736074
- DOI: 10.1038/s41598-025-86279-2
Deep brain stimulation of the anterior cingulate cortex reduces opioid addiction in preclinical studies
Abstract
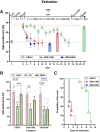
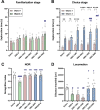
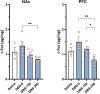
Substance Use Disorder (SUD) is a medical condition where an individual compulsively misuses drugs or alcohol despite knowing the negative consequences. The anterior cingulate cortex (ACC) has been implicated in various types of SUDs, including nicotine, heroin, and alcohol use disorders. Our research aimed to investigate the effects of deep brain stimulation (DBS) in the ACC as a potential therapeutic approach for morphine use disorder. Additionally, we measured c-Fos protein expression as an indicator of neural activity in the nucleus accumbens (NAc) and prefrontal cortex (PFC). Our findings indicate that high-frequency (130 Hz) DBS at different amperages, 150 µA and 200 µA in the ACC during the acquisition phase of morphine conditioned place preference (CPP) inhibited the rewarding properties of morphine. Furthermore, DBS at these intensities during the extinction phase facilitated the extinction and mitigated the reinstatement of morphine CPP triggered by drug priming. Morphine conditioning was associated with impaired novel object conditioning (NOR) and locomotor activity. While DBS in the acquisition and extinction phases at both intensities restored NOR memory, only DBS at 200 µA recovered locomotor activity in the open field test. Treatment with DBS at 200 µA decreased c-Fos protein expression in the NAc and PFC (compared to morphine-only group). In conclusion, our data indicate an intensity-dependent effect of ACC DBS on the acquisition, extinction, and reinstatement of morphine-induced CPP in rats. These findings suggest that ACC DBS could be a potential intervention for the treatment of morphine use disorder.
Keywords: Addiction; Anterior cingulate cortex; Deep brain stimulation; Morphine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Strecher, V. Internet methods for delivering behavioral and health-related interventions (eHealth). Annu. Rev. Clin. Psychol.3, 53–76 (2007). - PubMed
-
- Bottlender, M. & Soyka, M. Impact of craving on alcohol relapse during, and 12 months following, outpatient treatment. Alcohol Alcohol.39 (4), 357–361 (2004). - PubMed
-
- Cooney, N. L. et al. Alcohol cue reactivity, negative-mood reactivity, and relapse in treated alcoholic men. J. Abnorm. Psychol.106 (2), 243 (1997). - PubMed
-
- Seo, D. & Sinha, R. The neurobiology of alcohol craving and relapse. Handb. Clin. Neurol.125, 355–368 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

