Dynamic PRC1-CBX8 stabilizes a porous structure of chromatin condensates
- PMID: 39815045
- PMCID: PMC11919719
- DOI: 10.1038/s41594-024-01457-6
Dynamic PRC1-CBX8 stabilizes a porous structure of chromatin condensates
Abstract
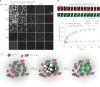
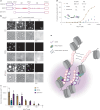
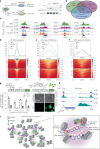
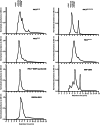
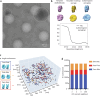
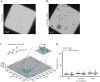
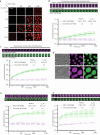
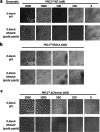
The compaction of chromatin is a prevalent paradigm in gene repression. Chromatin compaction is commonly thought to repress transcription by restricting chromatin accessibility. However, the spatial organization and dynamics of chromatin compacted by gene-repressing factors are unknown. Here, using cryo-electron tomography, we solved the three-dimensional structure of chromatin condensed by the polycomb repressive complex 1 (PRC1) in a complex with CBX8. PRC1-condensed chromatin is porous and stabilized through multivalent dynamic interactions of PRC1 with chromatin. Mechanistically, positively charged residues on the internally disordered regions of CBX8 mask negative charges on the DNA to stabilize the condensed state of chromatin. Within condensates, PRC1 remains dynamic while maintaining a static chromatin structure. In differentiated mouse embryonic stem cells, CBX8-bound chromatin remains accessible. These findings challenge the idea of rigidly compacted polycomb domains and instead provide a mechanistic framework for dynamic and accessible PRC1-chromatin condensates.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no conflicts of interest.
Figures




Update of
-
Dynamic PRC1-CBX8 stabilizes a porous structure of chromatin condensates.bioRxiv [Preprint]. 2024 Jul 17:2023.05.08.539931. doi: 10.1101/2023.05.08.539931. bioRxiv. 2024. Update in: Nat Struct Mol Biol. 2025 Mar;32(3):520-530. doi: 10.1038/s41594-024-01457-6. PMID: 38405976 Free PMC article. Updated. Preprint.
References
-
- Schuettengruber, B., Bourbon, H.-M., Di Croce, L. & Cavalli, G. Genome regulation by polycomb and trithorax: 70 years and counting. Cell171, 34–57 (2017). - PubMed
-
- Francis, N. J., Kingston, R. E. & Woodcock, C. L. Chromatin compaction by a polycomb group protein complex. Science306, 1574–1577 (2004). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

