A ROS-responsive hydrogel encapsulated with matrix metalloproteinase-13 siRNA nanocarriers to attenuate osteoarthritis progression
- PMID: 39815302
- PMCID: PMC11737235
- DOI: 10.1186/s12951-024-03046-7
A ROS-responsive hydrogel encapsulated with matrix metalloproteinase-13 siRNA nanocarriers to attenuate osteoarthritis progression
Abstract
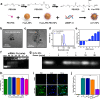
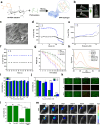
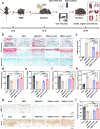
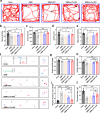
RNA interference (RNAi) and oxidative stress inhibition therapeutic strategies have been extensively utilized in the treatment of osteoarthritis (OA), the most prevalent degenerative joint disease. However, the synergistic effects of these approaches on attenuating OA progression remain largely unexplored. In this study, matrix metalloproteinase-13 siRNA (siMMP-13) was incorporated onto polyethylenimine (PEI)-polyethylene glycol (PEG) modified Fe3O4 nanoparticles, forming a nucleic acid nanocarrier termed si-Fe NPs. Subsequently, a poly(vinyl alcohol) (PVA) crosslinked phenylboronic acid (PBA)-modified hyaluronic acid (HA) hydrogel (HPP) was used to encapsulate the si-Fe NPs, resulting in a bifunctional hydrogel (si-Fe-HPP) with reactive oxygen species (ROS)-responsive and RNAi therapeutic properties. Studies in vitro demonstrated that si-Fe-HPP exhibited excellent biocompatibility, anti-inflammatory effects and prolonged stable retention time in knee joint. Intra-articular injection of si-Fe-HPP significantly attenuated cartilage degradation in mice with destabilization of the medial meniscus (DMM)-induced OA. The si-Fe-HPP treatment not only notably alleviated synovitis, osteophyte formation and subchondral bone sclerosis, but also markedly improved physical activity and reduced pain in DMM-induced OA mice. This study reveals that si-Fe-HPP, with its ROS-responsive and RNAi abilities, can significantly protect chondrocytes and attenuate OA progression, providing novel insights and directions for the development of therapeutic materials for OA treatment.
Keywords: Metalloproteinase-13 siRNA nanocarrier; Osteoarthritis; ROS-responsive Hydrogel.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were conducted with the approval of the Ethics Committee of Drum Tower Hospital, affiliated with Nanjing University. Consent for publication: All authors of this study agreed to publish. Competing interests: The authors declare no competing interests.
Figures



Similar articles
-
Injectable hydrogel encapsulating siMMP13 with anti-ROS and anti-apoptotic functions for osteoarthritis treatment.J Nanobiotechnology. 2024 Aug 2;22(1):466. doi: 10.1186/s12951-024-02740-w. J Nanobiotechnology. 2024. PMID: 39095867 Free PMC article.
-
Gel@CAT-L hydrogel mediates mitochondrial unfolded protein response to regulate reactive oxygen species and mitochondrial homeostasis in osteoarthritis.Biomaterials. 2025 Oct;321:123283. doi: 10.1016/j.biomaterials.2025.123283. Epub 2025 Mar 21. Biomaterials. 2025. PMID: 40222260
-
Hyaluronic acid composite hydrogel with enhanced lubrication and controllable drug release for the mitigation of osteoarthritis.Int J Biol Macromol. 2025 May;308(Pt 3):142677. doi: 10.1016/j.ijbiomac.2025.142677. Epub 2025 Mar 29. Int J Biol Macromol. 2025. PMID: 40164244
-
Dynamic hyaluronic acid hydrogel with covalent linked gelatin as an anti-oxidative bioink for cartilage tissue engineering.Biofabrication. 2021 Dec 31;14(1). doi: 10.1088/1758-5090/ac42de. Biofabrication. 2021. PMID: 34905737
-
Advanced application of stimuli-responsive drug delivery system for inflammatory arthritis treatment.Mater Today Bio. 2022 Feb 21;14:100223. doi: 10.1016/j.mtbio.2022.100223. eCollection 2022 Mar. Mater Today Bio. 2022. PMID: 35243298 Free PMC article. Review.
Cited by
-
Genetically engineered chondrocyte-mimetic nanoplatform attenuates osteoarthritis by blocking IL-1β and restoring sirtuin-3.Sci Adv. 2025 Jul 25;11(30):eadv4238. doi: 10.1126/sciadv.adv4238. Epub 2025 Jul 25. Sci Adv. 2025. PMID: 40712009 Free PMC article.
-
Preparing the functional biomaterial with osteogenic bioactivities by incorporating annealing pretreated silk fiber and iron oxide nanoparticles.Front Bioeng Biotechnol. 2025 Apr 10;13:1584081. doi: 10.3389/fbioe.2025.1584081. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40276031 Free PMC article.
-
Advances in locally administered nucleic acid therapeutics.Bioact Mater. 2025 Mar 10;49:218-254. doi: 10.1016/j.bioactmat.2025.02.043. eCollection 2025 Jul. Bioact Mater. 2025. PMID: 40144794 Free PMC article. Review.
-
Human Amniotic Epithelial Stem Cell Exosomes Regulate Chondrocyte Ferroptosis through ACTA2-AS1-Targeted Binding to ACSL4 for Osteoarthritis Intervention.Research (Wash D C). 2025 Aug 8;8:0814. doi: 10.34133/research.0814. eCollection 2025. Research (Wash D C). 2025. PMID: 40785968 Free PMC article.
References
-
- Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, Carr AJ. Osteoarthritis. Lancet. 2015;386:376–87. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

