Helminth extracellular vesicles co-opt host monocytes to drive T cell anergy
- PMID: 39815783
- PMCID: PMC11735955
- DOI: 10.1002/jev2.70027
Helminth extracellular vesicles co-opt host monocytes to drive T cell anergy
Abstract
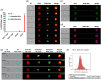
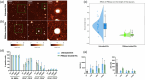
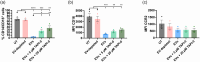
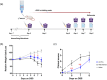
Parasitic helminths secrete extracellular vesicles (EVs) into their host tissues to modulate immune responses, but the underlying mechanisms are poorly understood. We demonstrate that Ascaris EVs are efficiently internalised by monocytes in human peripheral blood mononuclear cells and increase the percentage of classical monocytes. Furthermore, EV treatment of monocytes induced a novel anti-inflammatory phenotype characterised by CD14+, CD16-, CC chemokine receptor 2 (CCR2-) and programmed death-ligand 1 (PD-L1)+ cells. In addition, Ascaris EVs induced T cell anergy in a monocyte-dependent mechanism. Targeting professional phagocytes to induce both direct and indirect pathways of immune modulation presents a highly novel and efficient mechanism of EV-mediated host-parasite communication. Intra-peritoneal administration of EVs induced protection against gut inflammation in the dextran sodium sulphate model of colitis in mice. Ascaris EVs were shown to affect circulating immune cells and protect against gut inflammation; this highlights their potential as a subject for further investigation in inflammatory conditions driven by dysregulated immune responses. However, their clinical translation would require further studies and careful consideration of ethical implications.
Keywords: Ascaris; colitis; extracellular vesicles; helminth; host‐parasite interaction; immune modulation; inflammatory bowel disease; monocytes.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Ben Ami Pilo, H. , Khan Khilji, S. , Lühle, J. , Biskup, K. , Levy Gal, B. , Rosenhek Goldian, I. , Alfandari, D. , Revach, O. R.‐Y. , Kiper, E. , Morandi, M. I. , Rotkopf, R. , Porat, Z. , Blanchard, V. , Seeberger, P. H. , Regev‐Rudzki, N. , & Moscovitz, O. (2022). Sialylated N ‐glycans mediate monocyte uptake of extracellular vesicles secreted from Plasmodium falciparum ‐infected red blood cells. Journal of Extracellular Biology, 1, e33. - PMC - PubMed
-
- Bennett, F. , Luxenberg, D. , Ling, V. , Wang, I.‐M. , Marquette, K. , Lowe, D. , Khan, N. , Veldman, G. , Jacobs, K. A. , Valge‐Archer, V. E. , Collins, M. , & Carreno, B. M. (2003). Program death‐1 engagement upon TCR activation has distinct effects on costimulation and cytokine‐driven proliferation: Attenuation of ICOS, IL‐4, and IL‐21, But Not CD28, IL‐7, and IL‐15 Responses. The Journal of Immunology, 170, 711–718. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

