Airway Mycobiota-Microbiota During Pulmonary Exacerbation of Cystic Fibrosis Patients: A Culture and Targeted Sequencing Study
- PMID: 39816006
- PMCID: PMC11736540
- DOI: 10.1111/myc.70024
Airway Mycobiota-Microbiota During Pulmonary Exacerbation of Cystic Fibrosis Patients: A Culture and Targeted Sequencing Study
Abstract
Background: The airways of patients with cystic fibrosis (pwCF) harbour complex fungal and bacterial microbiota involved in pulmonary exacerbations (PEx) and requiring antimicrobial treatment. Descriptive studies analysing bacterial and fungal microbiota concomitantly are scarce, especially using both culture and high-throughput-sequencing (HTS).
Objectives: We analysed bacterial-fungal microbiota and inter-kingdom correlations in two French CF centres according to clinical parameters and antimicrobial choices.
Methods: Forty-eight pwCF with PEx from Creteil (n = 24) and Lille (n = 24) CF centres were included over 2 years. Sputa were collected for culture and targeted-HTS (ITS2 and V3-V4 targets). Sequencing and culture data, along with clinical, radiological and treatment data, were analysed. Two-level stratified analysis was performed to study potential confounding factors (age, CF mutation, FEV1 and antibiotics) on the centre factor. Inter-kingdom correlations were analysed.
Results: Significant differences in the bacterial microbiota profile were found between centres (p-value = 0.03). For mycobiota, the taxonomic distribution and diversity were comparable. HTS provided concordant but more detailed information than culture and increased detection of main CF fungi (> 25% more positive samples for Aspergillus or Scedosporium). FEV1 and systemic antibiotic before PEx influenced bacterial microbiota, but no clinical association was found with the mycobiota. No inter-kingdom correlation between Pseudomonas and fungi was found.
Conclusions: Describing concomitant bacterial and fungal communities of pwCF at the beginning of PEx using culture and HTS shows greater diversity in HTS and better detection in case of low microbial load. Interesting inter-kingdom correlations were observed, requiring further research on larger cohorts to understand the potential microbial interactions.
Keywords: Aspergillus; Candida; Scedosporium apiospermum; colonization; cystic fibrosis; internal transcribed spacer; pneumonia.
© 2025 The Author(s). Mycoses published by Wiley‐VCH GmbH.
Conflict of interest statement
Over the past 5 years, F.B. has received grants from Astellas, payments for lectures from Mundipharma and Gilead and travel expenses from Pfizer, Mundipharma and Gilead. C.A. received congress grants from Gilead and Biosynex and a payment for a lecture from Pfizer. R.E. received consulting fees, congress grants and payments for lectures from GSK and Astra Zeneca and is on the advisory board of Astra Zeneca, Novartis and Sanofi. R.D. received payments for lectures from Shionogi and Beckton Dickinson. C.T. received travel and congress grants from GSK and Sanofi. L.D. received payment for a lecture from Gilead and travel/congress grants from Gilead and Viatris Medical. The other authors declare no conflicts of interest.
Figures


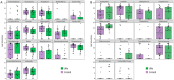
References
-
- la Mucoviscidose V., “Registre Français de la Mucoviscidose ‐ Bilan des Données,” (2022), https://www.vaincrelamuco.org/registredelamuco.
-
- Flume P. A. and VanDevanter D. R., “Cystic Fibrosis: Definition, Severity and Impact of Pulmonary Exacerbations,” in Acute Exacerbations of Pulmonary Diseases, eds. Burgel P. R., Contoli M., and López‐Campos J. L. (ERS Monograph. Sheffield: European Respiratory Society, 2017), 25–37.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

