Convergent-beam attosecond x-ray crystallography
- PMID: 39816474
- PMCID: PMC11731522
- DOI: 10.1063/4.0000275
Convergent-beam attosecond x-ray crystallography
Abstract
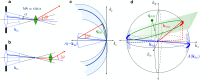
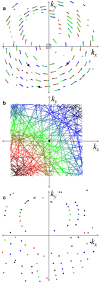
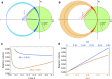
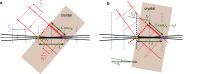
Sub-ångström spatial resolution of electron density coupled with sub-femtosecond to few-femtosecond temporal resolution is required to directly observe the dynamics of the electronic structure of a molecule after photoinitiation or some other ultrafast perturbation, such as by soft X-rays. Meeting this challenge, pushing the field of quantum crystallography to attosecond timescales, would bring insights into how the electronic and nuclear degrees of freedom couple, enable the study of quantum coherences involved in molecular dynamics, and ultimately enable these dynamics to be controlled. Here, we propose to reach this realm by employing convergent-beam x-ray crystallography with high-power attosecond pulses from a hard-x-ray free-electron laser. We show that with dispersive optics, such as multilayer Laue lenses of high numerical aperture, it becomes possible to encode time into the resulting diffraction pattern with deep sub-femtosecond precision. Each snapshot diffraction pattern consists of Bragg streaks that can be mapped back to arrival times and positions of X-rays on the face of a crystal. This can span tens of femtoseconds and can be finely sampled as we demonstrate experimentally. The approach brings several other advantages, such as an increase in the number of observable reflections in a snapshot diffraction pattern, all fully integrated, to improve the speed and accuracy of serial crystallography-especially for crystals of small molecules.
© 2025 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

References
-
- Dods R., Båth P., Morozov D., Gagnér V. A., Arnlund D., Luk H. L., Kübel J., Maj M., Vallejos A., Wickstrand C., Bosman R., Beyerlein K. R., Nelson G., Liang M., Milathianaki D., Robinson J., Harimoorthy R., Berntsen P., Malmerberg E., Johansson L., Andersson R., Carbajo S., Claesson E., Conrad C. E., Dahl P., Hammarin G., Hunter M. S., Li C., Lisova S., Royant A., Safari C., Sharma A., Williams G. J., Yefanov O., Westenhoff S., Davidsson J., DePonte D. P., Boutet S., Barty A., Katona G., Groenhof G., Brändén G., and Neutze R., “ Ultrafast structural changes within a photosynthetic reaction centre,” Nature 589, 310–314 (2021).10.1038/s41586-020-3000-7 - DOI - PubMed
-
- Pande K., Hutchison C. D. M., Groenhof G., Aquila A., Robinson J. S., Tenboer J., Basu S., Boutet S., DePonte D. P., Liang M., White T. A., Zatsepin N. A., Yefanov O., Morozov D., Oberthuer D., Gati C., Subramanian G., James D., Zhao Y., Koralek J., Brayshaw J., Kupitz C., Conrad C., Roy-Chowdhury S., Coe J. D., Metz M., Xavier P. L., Grant T. D., Koglin J. E., Ketawala G., Fromme R., Šrajer V., Henning R., Spence J. C. H., Ourmazd A., Schwander P., Weierstall U., Frank M., Fromme P., Barty A., Chapman H. N., Moffat K., van Thor J. J., and Schmidt M., “ Femtosecond structural dynamics drives the trans/cis isomerization in photoactive yellow protein,” Science 352, 725–729 (2016).10.1126/science.aad5081 - DOI - PMC - PubMed
-
- Nogly P., Weinert T., James D., Carbajo S., Ozerov D., Furrer A., Gashi D., Borin V., Skopintsev P., Jaeger K., Nass K., Båth P., Bosman R., Koglin J., Seaberg M., Lane T., Kekilli D., Brünle S., Tanaka T., Wu W., Milne C., White T., Barty A., Weierstall U., Panneels V., Nango E., Iwata S., Hunter M., Schapiro I., Schertler G., Neutze R., and Standfuss J., “ Retinal isomerization in bacteriorhodopsin captured by a femtosecond x-ray laser,” Science 361, eaat0094 (2018).10.1126/science.aat0094 - DOI - PubMed
LinkOut - more resources
Full Text Sources

