Effect of metabolic disorders on reactive gliosis and glial scarring at the early subacute phase of stroke in a mouse model of diabetes and obesity
- PMID: 39816479
- PMCID: PMC11733059
- DOI: 10.1016/j.ibneur.2024.12.002
Effect of metabolic disorders on reactive gliosis and glial scarring at the early subacute phase of stroke in a mouse model of diabetes and obesity
Abstract
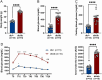
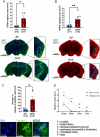
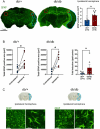
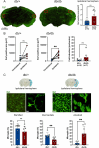
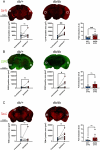
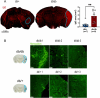
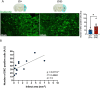
It is well recognized that type II Diabetes (T2D) and overweight/obesity are established risk factors for stroke, worsening also their consequences. However, the underlying mechanisms by which these disorders aggravate outcomes are not yet clear limiting the therapeutic opportunities. To fill this gap, we characterized, for the first time, the effects of T2D and obesity on the brain repair mechanisms occurring 7 days after stroke, notably glial scarring. In the present study, by performing a 30-minute middle cerebral artery occlusion (MCAO) on db/db (obese diabetics mice) and db/+ (controls) mice, we demonstrated that obese and diabetic mice displayed larger lesions (i.e. increased infarct volume, ischemic core, apoptotic cell number) and worsened neurological outcomes compared to their control littermates. We then investigated the formation of the glial scar in control and db/db mice 7 days post-stroke. Our observations argue in favor of a stronger and more persistent activation of astrocytes and microglia in db/db mice. Furthermore, an increased deposition of extracellular matrix (ECM) was observed in db/db vs control mice (i.e. chondroitin sulfate proteoglycan and collagen type IV). Consequently, we demonstrated for the first time that the db/db status is associated with increased astrocytic and microglial activation 7 days after stroke and resulted in higher deposition of ECM within the damaged area. Interestingly, the injury-induced neurogenesis appeared stronger in db/db as shown by the labeling of migrating neuroblast. This increase appeared correlated to the larger size of lesion. It nevertheless raises the question of the functional integration of the new neurons in db/db mice given the observed dense ECM, known to be repulsive for neuronal migration. Carefully limiting glial scar formation after stroke represents a promising area of research for reducing neuronal loss and limiting disability in diabetic/obese patients.
Keywords: CSPG; MCAO; astrogliosis; db/db mice; extracellular matrix; glial scar; gliosis; microgliosis.
© 2024 The Authors.
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Similar articles
-
Metabolic disorders exacerbate the formation of glial scar after stroke.Eur J Neurosci. 2024 Jun;59(11):3009-3029. doi: 10.1111/ejn.16325. Epub 2024 Apr 4. Eur J Neurosci. 2024. PMID: 38576159
-
M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice.Theranostics. 2021 Jan 1;11(3):1232-1248. doi: 10.7150/thno.48761. eCollection 2021. Theranostics. 2021. PMID: 33391532 Free PMC article.
-
Tenascin-C restricts reactive astrogliosis in the ischemic brain.Matrix Biol. 2022 Jun;110:1-15. doi: 10.1016/j.matbio.2022.04.003. Epub 2022 Apr 14. Matrix Biol. 2022. PMID: 35430380
-
Astrogliosis and glial scar in ischemic stroke - focused on mechanism and treatment.Exp Neurol. 2025 Mar;385:115131. doi: 10.1016/j.expneurol.2024.115131. Epub 2024 Dec 27. Exp Neurol. 2025. PMID: 39733853 Review.
-
Reactive astrogliosis in stroke: Contributions of astrocytes to recovery of neurological function.Neurochem Int. 2017 Jul;107:88-103. doi: 10.1016/j.neuint.2016.12.016. Epub 2017 Jan 3. Neurochem Int. 2017. PMID: 28057555 Review.
References
-
- Arzate D.M., Covarrubias L. Adult neurogenesis in the context of brain repair and functional relevance. Stem Cells Dev. 2020;29:544–554. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
