Postoperative analgesia with morphine promoting microglial activation and neuroinflammation induced by surgery aggravates perioperative neurocognitive dysfunction in aged mice
- PMID: 39816480
- PMCID: PMC11732693
- DOI: 10.1016/j.ibneur.2024.12.008
Postoperative analgesia with morphine promoting microglial activation and neuroinflammation induced by surgery aggravates perioperative neurocognitive dysfunction in aged mice
Abstract
Introduction: Perioperative neurocognitive dysfunction (PND) is a significant challenge for patients who need surgery worldwide. Morphine can trigger an intense inflammatory reaction in the central nervous system (CNS) at the same time as analgesia, thus adverse effects aggravating PND. Microglia polarization is closely involved in the regulation of neuroinflammation and the TLR4/MyD88/NF-κB signaling pathway. However, the mechanisms of morphine analgesia aggravating PND impairment remain unclear.
Methods: Tibial fracture surgery was performed in 18 months old male C57BL/6 J mice to mimic human orthopedic surgery and postoperative analgesia with morphine hypodermic or ropivacaine. Levels of inflammatory factors in the hippocampus, activation, and phenotype of microglia, an essential protein of TLR4/MyD88/NF-κB signal pathway, synaptic plasticity, and hippocampal-dependent memory function were evaluated after surgery and postoperative analgesia.
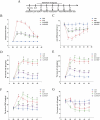
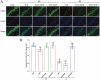
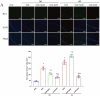
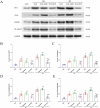
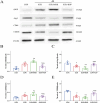
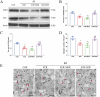
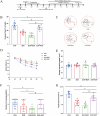
Results: Morphine postoperative analgesia increased the expression of pro-inflammatory cytokines IL-1 β, IL-6, and TNF-α, decreased the level of anti-inflammatory IL-10, aggravated the activation of microglia and the destruction of synaptic plasticity in the hippocampus, resulting in hippocampal neuron loss, a significant decrease in the number of synapses and cognitive impairment in aged mice. In addition, the aggravation of neuroinflammatory response and the activation of microglia may be mediated by TLR4/MyD88/NF- κ B signal pathway.
Conclusion: Our results demonstrate that morphine postoperative analgesia may aggravate microglia activation and neuroinflammation in the hippocampus by regulating the TLR4/MyD88/NF- κ B signal pathway and inhibiting the synaptic plasticity hippocampal neurons. It aggravated the acute cognitive decline and cognitive impairment after tibial fracture in elderly mice.
Keywords: Microglia activation; Morphine; Neuroinflammation; Perioperative neurocognitive dysfunction; Synaptic plasticity.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Free heme induces neuroinflammation and cognitive impairment by microglial activation via the TLR4/MyD88/NF-κB signaling pathway.Cell Commun Signal. 2024 Jan 5;22(1):16. doi: 10.1186/s12964-023-01387-8. Cell Commun Signal. 2024. PMID: 38183122 Free PMC article.
-
Fluoxetine alleviates postoperative cognitive dysfunction by attenuating TLR4/MyD88/NF-κB signaling pathway activation in aged mice.Inflamm Res. 2023 Jun;72(6):1161-1173. doi: 10.1007/s00011-023-01738-8. Epub 2023 May 15. Inflamm Res. 2023. PMID: 37188940
-
Nrf2 Ameliorates Sevoflurane-Induced Cognitive Deficits in Aged Mice by Inhibiting Neuroinflammation in the Hippocampus.Mol Neurobiol. 2025 Jun;62(6):8048-8064. doi: 10.1007/s12035-025-04777-w. Epub 2025 Feb 19. Mol Neurobiol. 2025. PMID: 39969679
-
Toll-Like Receptor 4 (TLR4)/Opioid Receptor Pathway Crosstalk and Impact on Opioid Analgesia, Immune Function, and Gastrointestinal Motility.Front Immunol. 2020 Jul 8;11:1455. doi: 10.3389/fimmu.2020.01455. eCollection 2020. Front Immunol. 2020. PMID: 32733481 Free PMC article. Review.
-
Research progress of microglial surface receptors in perioperative neurocognitive disorders.Ibrain. 2023 Nov 30;10(4):450-461. doi: 10.1002/ibra.12136. eCollection 2024 Winter. Ibrain. 2023. PMID: 39691417 Free PMC article. Review.
Cited by
-
Neuroprotective Effects of Qi Jing Wan and Its Active Ingredient Diosgenin Against Cognitive Impairment in Plateau Hypoxia.Pharmaceuticals (Basel). 2025 May 17;18(5):738. doi: 10.3390/ph18050738. Pharmaceuticals (Basel). 2025. PMID: 40430556 Free PMC article.
References
-
- Ding X., Gao X., Wang Z., Jiang X., Lu S., Xu J., Qin G., Gu Z., Huang D. Preoperative chronic and acute pain affects postoperative cognitive function mediated by neurotransmitters. J. Mol. Neurosci. 2021;71(3):515–526. - PubMed
-
- Evered L., Silbert B., Knopman D.S., Scott D.A., DeKosky S.T., Rasmussen L.S., Oh E.S., Crosby G., Berger M., Eckenhoff R.G. Nomenclature Consensus Working Group. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br. J. Anaesth. 2018;121(5):1005–1012. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
