Alpha-Lipoic Acid-Mediated Inhibition of LTB4 Synthesis Suppresses Epithelial-Mesenchymal Transition, Modulating Functional and Tumorigenic Capacities in Non-Small Cell Lung Cancer A549 Cells
- PMID: 39816494
- PMCID: PMC11731977
- DOI: 10.1016/j.curtheres.2024.100765
Alpha-Lipoic Acid-Mediated Inhibition of LTB4 Synthesis Suppresses Epithelial-Mesenchymal Transition, Modulating Functional and Tumorigenic Capacities in Non-Small Cell Lung Cancer A549 Cells
Abstract
Background: Leukotriene B4 (LTB4) plays a crucial role in carcinogenesis by inducing epithelial-mesenchymal transition (EMT), a process associated with tumor progression. The synthesis of LTB4 is mediated by leukotriene A4 hydrolase (LTA4H), and it binds to the receptors BLT1 and BLT2. Dysregulation in LTB4 production is linked to the development of various pathologies. Therefore, the identification or design of inhibitors of LTB4 synthesis or receptor antagonists represents an ongoing challenge. In this context, our laboratory previously demonstrated that alpha-lipoic acid (ALA) inhibits LTA4H. The objective of this study was to evaluate the effect of ALA on the expression of canonical EMT markers and the functional and tumorigenic capacities induced by LTB4 in A549 cells.
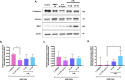
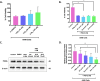
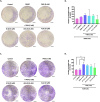
Methods: The expression of cPLA2, 5LOX, FLAP, LTA4H, BLT1, and LTB4 production in human adenocarcinomic alveolar basal epithelial A549 cells was assessed using Western blot, RT-qPCR, and ELISA, respectively. Subsequently, the expression of canonical EMT markers was evaluated by Western blot. Functional assays were performed to assess cell viability, proliferation, invasion, migration, and clonogenicity using MTT, Western blot, Transwell assays, and colony formation assays, respectively. Results were expressed as median with interquartile range (n≥3) and analyzed using the Kruskal-Wallis or Tukey multiple comparisons tests.
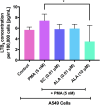
Results: A549 cells express key proteins involved in LTB4 synthesis and receptor binding, including LTA4H and BLT1, and ALA inhibits the production of LTB4. Additionally, LTA4H and BLT1 were detected in lung adenocarcinoma tissue samples. LTB4 was found to induce EMT, whereas ALA treatment enhanced the expression of epithelial markers and reduced the expression of mesenchymal markers. Furthermore, ALA treatment resulted in a decrease in LTB4 levels and attenuated the functional and tumorigenic capacities of A549 cells, including their viability, migration, invasion, and clonogenic potential.
Conclusions: These findings suggest that ALA may offer therapeutic potential in the context of lung cancer, as it could be integrated into conventional pharmacological therapies to enhance treatment efficacy and mitigate the adverse effects associated with chemotherapy. Further studies are warranted to confirm the clinical applicability of ALA as an adjunctive treatment in lung cancer.
Keywords: Alpha lipoic acid (ALA); Epithelial Mesenchymal Transition (EMT) and Functional and tumor capacities; Leukotriene A4 Hydrolase (LTA4H); Leukotriene B4 (LTB4); Small cell lung carcinoma and non-small cell lung cancer (NSCLC).
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures




References
-
- Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. Available from: https://gco.iarc.who.int/today, accessed [21st February, 2024].
-
- Colotta F., Allavena P., Sica A., et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. - PubMed
-
- O'Callaghan D.S., O'Donnell D., O'Connell F., et al. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5(12):2024–2036. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

