Metal-organic framework catalysed multicomponent reactions towards the synthesis of Pyrans
- PMID: 39816504
- PMCID: PMC11732708
- DOI: 10.1016/j.heliyon.2024.e41439
Metal-organic framework catalysed multicomponent reactions towards the synthesis of Pyrans
Abstract
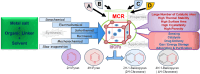
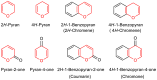
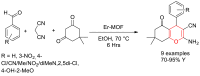
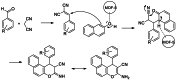
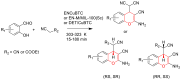
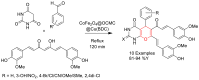
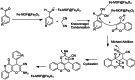
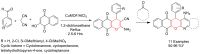
Metal-Organic Frameworks (MOFs) gaining increasing interest in heterogeneous catalysis owing to their advantageous properties such as superior porosity, high surface area, ample catalytic sites. Their properties can be tailored by varying the metal ions or metal clusters (nodes) and organic linkers. Magnetically active nano core-shell MOF composites are also discovered for easy separation and reuse of catalyst. MOF catalysed multicomponent reactions (MCRs) satisfy several green chemistry principles and thus can be considered as a sturdy step towards sustainable chemical synthesis. In this article, synthesis of biologically potent pyran scaffolds through MOF catalysed MCR approaches have been reviewed. Preparation of MOF catalyst, its catalytic performance in pyran synthesis, reusability has been discussed with reaction for each example.
Keywords: Heterogeneous catalysis; Metal organic framework; Multicomponent reactions; Pyran; Sustainable chemistry.
© 2025 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Corresponding author serving as an associate editor in Heliyon Chemistry. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

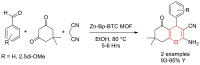
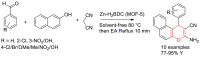
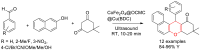

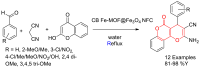
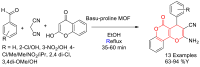
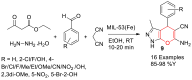
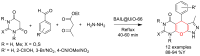
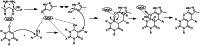

Similar articles
-
Site Isolation in Metal-Organic Frameworks Enables Novel Transition Metal Catalysis.Acc Chem Res. 2018 Sep 18;51(9):2129-2138. doi: 10.1021/acs.accounts.8b00297. Epub 2018 Aug 21. Acc Chem Res. 2018. PMID: 30129753
-
A review on multicomponent reactions catalysed by zero-dimensional/one-dimensional titanium dioxide (TiO2) nanomaterials: Promising green methodologies in organic chemistry.J Environ Manage. 2021 Feb 1;279:111603. doi: 10.1016/j.jenvman.2020.111603. Epub 2020 Nov 7. J Environ Manage. 2021. PMID: 33172705 Review.
-
Bi Metal-Organic Framework (Ce/Ni-BTC) as Heterogeneous Catalyst for the Green Synthesis of Substituted Chromeno[4, 3-b]quinolone under Solvent Free Condition.Curr Org Synth. 2021;18(5):475-482. doi: 10.2174/1570179418666210122100240. Curr Org Synth. 2021. PMID: 33480346
-
Programmable Logic in Metal-Organic Frameworks for Catalysis.Adv Mater. 2021 Nov;33(46):e2007442. doi: 10.1002/adma.202007442. Epub 2021 May 28. Adv Mater. 2021. PMID: 34050572 Review.
-
Multifunctional metal-organic framework catalysts: synergistic catalysis and tandem reactions.Chem Soc Rev. 2017 Jan 3;46(1):126-157. doi: 10.1039/c6cs00250a. Chem Soc Rev. 2017. PMID: 27841411 Review.
References
-
- Kaldre D. In: Comprehensive Heterocyclic Chemistry IV. StC Black D., Cossy J., Stevens C.V., editors. Vol. 7.09. Elsevier; Amsterdam, The Netherlands: 2022. Pyrans and benzo derivatives: applications; pp. 491–511. (Volume 7: Six-Membered Rings with One Heteroatom and Their Fused Carbocyclic, Derivatives).
-
- Masamune S., Castellucci N.T. γ-Pyran. J. Am. Chem. Soc. 1962;84:2452–2453.
-
- Moumou Y., Vasseur J., Trotin F., Dubois J. Catechin production by callus cultures of Fagopyrum esculentum, Phytochemistry. 1992;31:1239–1241.
-
- Soria-Mercado I.E., Prieto-Davo A., Jensen P.R., Fenical W.J. Antibiotic terpenoid chloro-dihydroquinones from a new marine actinomycete. J. Nat. Prod. 2005;68:904–910. - PubMed
-
- Gewali M.B., Tezuka Y., Banskota A.H., Ali M.S., Saiki I., Dong H., Kadota S., Epicalyxin F., Calyxin I. Two novel antiproliferative diarylheptanoids from the seeds of Alpinia blepharocalyx. org. Lett. 1999;1:1733–1736. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

