Hidden in plain sight: How helminths manage to thrive in host blood
- PMID: 39816845
- PMCID: PMC11732017
- DOI: 10.3389/fpara.2023.1128299
Hidden in plain sight: How helminths manage to thrive in host blood
Abstract
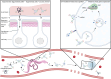
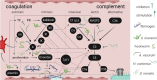
Parasitic helminths have evolved a plethora of elegant stratagems to regulate and evade the host immune system, contributing to their considerable persistence and longevity in their vertebrate hosts. Various mechanisms to achieve this state have been described, ranging from interfering with or actively modulating host immune responses to hiding from immune recognition. Because they damage surrounding vessels and disturb blood flow, blood-borne and blood-feeding parasites in particular must deal with much more than immune effector cells. Management of the host complement system and coagulation cascade, as well as the development of processes of hiding and masking, represent hallmarks of life in blood. Here we review recent findings on putative evasion strategies employed by blood-borne parasitic helminths, focusing on the interaction with and utilisation of host serum components by nematodes and trematodes.
Keywords: blood-borne; coagulation; complement system; helminth; host factors; immune evasion; innate immunity.
Copyright © 2023 Dagenais and Tritten.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bennett A. P., de la Torre-Escudero E., Dermott S. S., Threadgold L. T., Hanna R. E., Robinson M. W. (2022). Fasciola hepatica gastrodermal cells selectively release extracellular vesicles via a novel atypical secretory mechanism. Int. J. Mol. Sci. 23, 5525. doi: 10.3390/ijms23105525 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

