Photodegradation of naphthalene-derived particle oxidation products
- PMID: 39816852
- PMCID: PMC11727846
- DOI: 10.1039/d4ea00125g
Photodegradation of naphthalene-derived particle oxidation products
Abstract
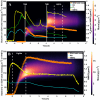
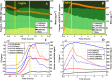
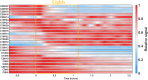
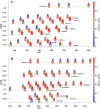
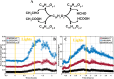
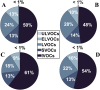
While photochemical aging is known to alter secondary organic aerosol (SOA) properties, this process remains poorly constrained for anthropogenic SOA. This study investigates the photodegradation of SOA produced from the hydroxyl radical-initiated oxidation of naphthalene under low- and high-NO x conditions. We used state-of-the-art mass spectrometry (MS) techniques, including extractive electrospray ionization and chemical ionization MS, for the in-depth molecular characterization of gas and particulate phases. SOA were exposed to simulated irradiation at different stages, i.e., during formation and growth. We found a rapid (i.e. >30 min) photodegradation of high-molecular-weight compounds in the particle-phase. Notably, species with 20 carbon atoms (C20) decreased by 2/3 in the low-NO x experiment which was associated with particle mass loss (∼12%). Concurrently, the formation of oligomers with shorter carbon skeletons in the particle-phase was identified along with the release of volatile products such as formic acid and formaldehyde in the gas-phase. These reactions are linked to photolabile functional groups within the naphthalene-derived SOA products, which increases their likelihood of being degraded under UV light. Overall, photodegradation caused a notable change in the molecular composition altering the physical properties (e.g., volatility) of naphthalene-derived SOA.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Burnett R. Chen H. Szyszkowicz M. Fann N. Hubbell B. Pope C. A. Apte J. S. Brauer M. Cohen A. Weichenthal S. Coggins J. Di Q. Brunekreef B. Frostad J. Lim S. S. Kan H. Walker K. D. Thurston G. D. Hayes R. B. Lim C. C. Turner M. C. Jerrett M. Krewski D. Gapstur S. M. Diver W. R. Ostro B. Goldberg D. Crouse D. L. Martin R. V. Peters P. Pinault L. Tjepkema M. Van Donkelaar A. Villeneuve P. J. Miller A. B. Yin P. Zhou M. Wang L. Janssen N. A. H. Marra M. Atkinson R. W. Tsang H. Quoc Thach T. Cannon J. B. Allen R. T. Hart J. E. Laden F. Cesaroni G. Forastiere F. Weinmayr G. Jaensch A. Nagel G. Concin H. Spadaro J. V. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. U. S. A. 2018;115:9592–9597. doi: 10.1073/pnas.1803222115. - DOI - PMC - PubMed
-
- Shiraiwa M. Ueda K. Pozzer A. Lammel G. Kampf C. J. Fushimi A. Enami S. Arangio A. M. Fröhlich-Nowoisky J. Fujitani Y. Furuyama A. Lakey P. S. J. Lelieveld J. Lucas K. Morino Y. Pöschl U. Takahama S. Takami A. Tong H. Weber B. Yoshino A. Sato K. Aerosol Health Effects from Molecular to Global Scales. Environ. Sci. Technol. 2017;51:13545–13567. doi: 10.1021/acs.est.7b04417. - DOI - PubMed
-
- Déméautis T. Delles M. Tomaz S. Monneret G. Glehen O. Devouassoux G. George C. Bentaher A. Pathogenic Mechanisms of Secondary Organic Aerosols. Chem. Res. Toxicol. 2022;35(7):1146–1161. - PubMed
-
- Haywood J. Boucher O. Estimates of the direct and indirect radiative forcing due to tropospheric aerosols: A review. Rev. Geophys. 2000;38:513–543. doi: 10.1029/1999RG000078. - DOI
LinkOut - more resources
Full Text Sources
