Characterization of preclinical models to investigate spinal cord stimulation for neuropathic pain: a systematic review and meta-analysis
- PMID: 39816902
- PMCID: PMC11732658
- DOI: 10.1097/PR9.0000000000001228
Characterization of preclinical models to investigate spinal cord stimulation for neuropathic pain: a systematic review and meta-analysis
Abstract
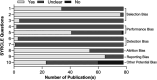
Despite advancements in preclinical and clinical spinal cord stimulation (SCS) research, the mechanisms of SCS action remain unclear. This may result from challenges in translatability of findings between species. Our systematic review (PROSPERO: CRD42023457443) aimed to comprehensively characterize the important translational components of preclinical SCS models, including stimulating elements and stimulation specifications. Databases (Embase, PubMed, Web of Science, and WikiStim) were searched on October 5, 2023, identifying 78 studies meeting the search criteria. We conducted a post hoc meta-analysis, including subgroup analyses and meta-regression, to assess SCS efficacy on mechanical hypersensitivity in rats subjected to neuropathic pain. Although monopolar electrodes were predominantly used as stimulating elements until 2013, quadripolar paddle and cylindrical leads gained recent popularity. Most research was conducted using 50 Hz and 200 µs stimulation. Motor threshold (MT) estimation was the predominant strategy to determine SCS intensity, which was set to 71.9% of MT on average. Our analysis revealed a large effect size for SCS (Hedge g = 1.13, 95% CI: [0.93, 1.32]) with similar magnitudes of effect between conventional (≤100 Hz) and nonconventional SCS paradigms while sham SCS had nonsignificant effect size. In addition, different stimulation intensity, frequency, and electrode design did not affect effect size. The risk of bias was assessed using Systematic Review Centre for Laboratory animal Experimentation criteria and was unclear, and only the frequency subgroup analysis showed publication bias. In summary, our review characterizes the critical components of preclinical SCS models and provides recommendations to improve reproducibility and translatability, thereby advancing the scientific foundation for SCS research.
Keywords: Analgesia; Mechanical hypersensitivity; Neuropathic pain; Rat; Spinal cord stimulation.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The International Association for the Study of Pain.
Conflict of interest statement
The authors have no conflict of interest to declare.Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures




Similar articles
-
Ninety-Hz Spinal Cord Stimulation-Induced Analgesia Is Dependent on Active Charge Balance and Is Nonlinearly Related to Amplitude: A Sham-Controlled Behavioral Study in a Rodent Model of Chronic Neuropathic Pain.Neuromodulation. 2024 Jan;27(1):95-107. doi: 10.1016/j.neurom.2023.09.005. Epub 2023 Nov 18. Neuromodulation. 2024. PMID: 37978974
-
Conventional-SCS vs. Burst-SCS and the Behavioral Effect on Mechanical Hypersensitivity in a Rat Model of Chronic Neuropathic Pain: Effect of Amplitude.Neuromodulation. 2018 Jan;21(1):19-30. doi: 10.1111/ner.12731. Epub 2017 Nov 27. Neuromodulation. 2018. PMID: 29178358
-
Spinal Cord Stimulation Paradigms and Alleviation of Neuropathic Pain Behavior in Experimental Painful Diabetic Polyneuropathy.Neuromodulation. 2024 Dec;27(8):1330-1337. doi: 10.1016/j.neurom.2024.06.007. Epub 2024 Jul 20. Neuromodulation. 2024. PMID: 39033461
-
Parameters of Spinal Cord Stimulation in Complex Regional Pain Syndrome: Systematic Review and Meta-analysis of Randomized Controlled Trials.Pain Physician. 2022 Nov;25(8):521-530. Pain Physician. 2022. PMID: 36375180
-
Does industry funding and study location impact findings from randomized controlled trials of spinal cord stimulation? A systematic review and meta-analysis.Reg Anesth Pain Med. 2024 Apr 2;49(4):272-284. doi: 10.1136/rapm-2023-104674. Reg Anesth Pain Med. 2024. PMID: 37611944
Cited by
-
Emerging targets and translational challenges in treating paclitaxel-induced peripheral neuropathy.Mol Biol Rep. 2025 Aug 18;52(1):833. doi: 10.1007/s11033-025-10938-w. Mol Biol Rep. 2025. PMID: 40824316 Review.
References
-
- Austin PJ, Moalem-Taylor G. Animal models of neuropathic pain due to nerve injury. In: Pilowsky PM, Farnham MMJ, Fong AY, editors. Stimulation and Inhibition of Neurons. Neuromethods. Vol. 78. Totowa, NJ: Humana Press, 2013. p. 239–60.
-
- Barchini J, Tchachaghian S, Shamaa F, Jabbur SJ, Meyerson BA, Song Z, Linderoth B, Saadé NE. Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience 2012;215:196–208. - PubMed
-
- van Beek M, Hermes D, Honig WM, Linderoth B, van Kuijk SMJ, van Kleef M, Joosten EA. Long-term spinal cord stimulation alleviates mechanical hypersensitivity and increases peripheral cutaneous blood perfusion in experimental painful diabetic polyneuropathy. Neuromodulation 2018;21:472–9. - PMC - PubMed
-
- Boston Scientific . Percutaneous leads—directions for use. Boston Scientific; https://www.bostonscientific.com/elabeling/ie/en/home.html (2024, Accessed February 1, 2024).
Publication types
LinkOut - more resources
Full Text Sources
