Valvular Heart Disease-Related Mortality Between Middle- and High-Income Countries During 2000 to 2019
- PMID: 39817085
- PMCID: PMC11733992
- DOI: 10.1016/j.jacadv.2024.101133
Valvular Heart Disease-Related Mortality Between Middle- and High-Income Countries During 2000 to 2019
Abstract
Background: Valvular heart disease (VHD) management has evolved rapidly in recent decades, but disparities in health care access persist among countries with varying socioeconomic backgrounds.
Objectives: The purpose of this study was to investigate global mortality trends from VHD and assess the difference between middle- and high-income countries.
Methods: We obtained mortality data from the World Health Organization Mortality Database for VHD and its subgroups (rheumatic valvular disease [RVD], infective endocarditis [IE], aortic stenosis [AS], and mitral regurgitation [MR]) from 2000 to 2019. Age-specific and age-standardized mortality rates per 100,000 persons in middle- and high-income countries were calculated, and trends were analyzed using joinpoint regression.
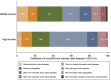
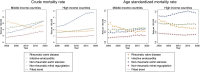
Results: A total of 93 countries (42 middle-income and 51 high-income) were included in the analysis. Both middle- and high-income countries showed an increasing trend in crude VHD mortality rate. In middle-income countries, the age-standardized VHD-related mortality rate was constant (0.0%/year), with decreasing RVD (-2.7%/year) and increasing IE, AS, and MR (0.8%/year, 2.0%/year, and 2.2%/year, respectively). In high-income countries, the age-standardized VHD-related mortality rate was decreasing (-0.6%/year). However, there was a rapid increase in mortality rate from IE in age ≤39 years after 2009 (7.0%/year). Moreover, there was a decreasing mortality rate from AS after 2015 but an increasing rate from MR after 2013, particularly in age ≥80 years.
Conclusions: Our study identified a rising burden of VHD-related mortality worldwide. The distribution and trends of VHD mortality differed between middle- and high-income countries. Further investigation is needed to understand the underlying etiology of these varying mortality trends in VHD and its subgroups.
Keywords: global mortality trends; national income levels; valvular heart disease.
© 2024 The Authors.
Conflict of interest statement
Dr Halkos is on the advisory board for Medtronic. Dr Grubb is on the advisory board for Medtronic, Boston Scientific, Abbott, and 4C Medical; and is a consultant for or received an honorarium from Medtronic, Boston Scientific, Abbott, and 4C Medical and Edwards Lifesciences. Dr Bhatt is on the advisory board for Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Stasys; is on the Board of Directors for American Heart Association New York City, Angiowave (stock options), Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock); is a consultant for Broadview Ventures, Hims, SFJ, Youngene; is on the Data Monitoring Committees for Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by 10.13039/100006279St. Jude Medical, now 10.13039/100000046Abbott), 10.13039/100008497Boston Scientific (Chair, PEITHO trial), Cleveland Clinic, Contego Medical (Chair, PERFORMANCE 2), 10.13039/100006513Duke Clinical Research Institute, 10.13039/100000871Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by 10.13039/501100022274Daiichi Sankyo; for the ABILITY-DM trial, funded by 10.13039/100030895Concept Medical; for ALLAY-HF, funded by Alleviant Medical), 10.13039/100008272Novartis, 10.13039/100030936Population Health Research Institute; Rutgers University (for the 10.13039/100000002NIH-funded MINT Trial); has received honoraria from American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by 10.13039/100001003Boehringer Ingelheim; AEGIS-II executive committee funded by 10.13039/100008322CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, 10.13039/100006513Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by 10.13039/501100004914Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by 10.13039/100004326Bayer), WebMD (CME steering committees), Wiley (steering committee); other services from Clinical Cardiology (Deputy Editor); holds a patent on sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon; neither I nor Brigham and Women's Hospital receive any income from this patent); has received research funding from 10.13039/100000046Abbott, Acesion Pharma, Afimmune, Aker Biomarine, 10.13039/100006400Alnylam, Amarin, 10.13039/100002429Amgen, 10.13039/100004325AstraZeneca, 10.13039/100015340Bayer, Beren, 10.13039/100001003Boehringer Ingelheim, 10.13039/100008497Boston Scientific, 10.13039/100002491Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, 10.13039/100007560Chiesi, CinCor, Cleerly, 10.13039/100008322CSL Behring, 10.13039/501100023368Eisai, 10.13039/100009933Ethicon, Faraday Pharmaceuticals, 10.13039/501100004914Ferring Pharmaceuticals, 10.13039/100005632Forest Laboratories, Fractyl, Garmin, 10.13039/501100018679HLS Therapeutics, 10.13039/501100016198Idorsia, 10.13039/100010721Ironwood, Ischemix, 10.13039/100005565Janssen, Javelin, Lexicon, 10.13039/100004312Lilly, 10.13039/100004374Medtronic, 10.13039/100004334Merck, 10.13039/100019533Moderna, 10.13039/100016619MyoKardia, NirvaMed, 10.13039/100008272Novartis, 10.13039/501100004191Novo Nordisk, 10.13039/100019120Otsuka, Owkin, 10.13039/100004319Pfizer, PhaseBio, PLx Pharma, Recardio, 10.13039/100009857Regeneron, Reid Hoffman Foundation, 10.13039/100004337Roche, 10.13039/100004339Sanofi, Stasys, Synaptic, 10.13039/100015237The Medicines Company, Youngene, 89Bio; has received royalties from Elsevier (Editor, Braunwald’s Heart Disease); is a site co-investigator for Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, Vascular Solutions; is a trustee for American College of Cardiology; and reports unfunded research from FlowCo. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- Coffey S., Roberts-Thomson R., Brown A., et al. Global epidemiology of valvular heart disease. Nat Rev Cardiol. 2021;18:853–864. - PubMed
-
- Vaduganathan M., Mensah G.A., Turco J.V., Fuster V., Roth G.A. The global burden of cardiovascular diseases and risk: a compass for future Health. J Am Coll Cardiol. 2022;80:2361–2371. - PubMed
-
- Tibazarwa K.B., Volmink J.A., Mayosi B.M. Incidence of acute rheumatic fever in the world: a systematic review of population-based studies. Heart. 2008;94:1534–1540. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

