Clonal analysis of SepSecS-specific B and T cells in autoimmune hepatitis
- PMID: 39817450
- PMCID: PMC11735102
- DOI: 10.1172/JCI183776
Clonal analysis of SepSecS-specific B and T cells in autoimmune hepatitis
Abstract
Autoimmune hepatitis (AIH) is a rare chronic inflammatory liver disease characterized by the presence of autoantibodies, including those targeting O-phosphoseryl-tRNA:selenocysteine-tRNA synthase (SepSecS), also known as soluble liver antigen (SLA). Anti-SepSecS antibodies have been associated with a more severe phenotype, suggesting a key role for the SepSecS autoantigen in AIH. To analyze the immune response to SepSecS in patients with AIH at the clonal level, we combined sensitive high-throughput screening assays with the isolation of monoclonal antibodies (mAbs) and T cell clones. The anti-SepSecS mAbs isolated were primarily IgG1, affinity-matured compared with their germline versions, and recognized at least 3 nonoverlapping epitopes. SepSecS-specific CD4+ T cell clones were found in patients with AIH who were anti-SLA-positive and anti-SLA-negative,and, to a lesser extent, in patients with non-AIH liver diseases and in healthy individuals. SepSecS-specific T cell clones from patients with AIH produced IFN-γ, IL-4, and IL-10, targeted multiple SepSecS epitopes, and, in one patient, were clonally expanded in both blood and liver biopsy. Finally, SepSecS-specific B cell clones, but not those of unrelated specificities, were able to present soluble SepSecS to specific T cells. Collectively, our study provides the first detailed analysis of B and T cell repertoires targeting SepSecS in patients with AIH, offering a rationale for improved targeted therapies.
Keywords: Autoimmunity; Hepatitis; Immunoglobulins; Immunology; T cells.
Figures

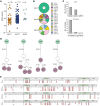
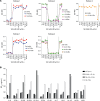


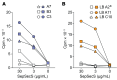
Comment in
- Elucidating the role of autoreactive T cells and B cells in autoimmune hepatitis
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

