Survival strategies of cancer cells: the role of macropinocytosis in nutrient acquisition, metabolic reprogramming, and therapeutic targeting
- PMID: 39817564
- PMCID: PMC11925119
- DOI: 10.1080/15548627.2025.2452149
Survival strategies of cancer cells: the role of macropinocytosis in nutrient acquisition, metabolic reprogramming, and therapeutic targeting
Abstract
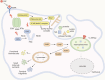
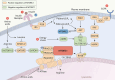
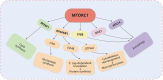
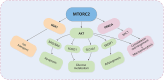
Macropinocytosis is a nonselective form of endocytosis that allows cancer cells to largely take up the extracellular fluid and its contents, including nutrients, growth factors, etc. We first elaborate meticulously on the process of macropinocytosis. Only by thoroughly understanding this entire process can we devise targeted strategies against it. We then focus on the central role of the MTOR (mechanistic target of rapamycin kinase) complex 1 (MTORC1) in regulating macropinocytosis, highlighting its significance as a key signaling hub where various pathways converge to control nutrient uptake and metabolic processes. The article covers a comprehensive analysis of the literature on the molecular mechanisms governing macropinocytosis, including the initiation, maturation, and recycling of macropinosomes, with an emphasis on how these processes are hijacked by cancer cells to sustain their growth. Key discussions include the potential therapeutic strategies targeting macropinocytosis, such as enhancing drug delivery via this pathway, inhibiting macropinocytosis to starve cancer cells, blocking the degradation and recycling of macropinosomes, and inducing methuosis - a form of cell death triggered by excessive macropinocytosis. Targeting macropinocytosis represents a novel and innovative approach that could significantly advance the treatment of cancers that rely on this pathway for survival. Through continuous research and innovation, we look forward to developing more effective and safer anti-cancer therapies that will bring new hope to patients.Abbreviation: AMPK: AMP-activated protein kinase; ASOs: antisense oligonucleotides; CAD: carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase; DC: dendritic cell; EGF: epidermal growth factor; EGFR: epidermal growth factor receptor; ERBB2: erb-b2 receptor tyrosine kinase 2; ESCRT: endosomal sorting complex required for transport; GAP: GTPase-activating protein; GEF: guanine nucleotide exchange factor; GRB2: growth factor receptor bound protein 2; LPP: lipopolyplex; MTOR: mechanistic target of rapamycin kinase; MTORC1: mechanistic target of rapamycin kinase complex 1; MTORC2: mechanistic target of rapamycin kinase complex 2; NSCLC: non-small cell lung cancer; PADC: pancreatic ductal adenocarcinoma; PDPK1: 3-phosphoinositide dependent protein kinase 1; PI3K: phosphoinositide 3-kinase; PIK3C3: phosphatidylinositol 3-kinase catalytic subunit type 3; PtdIns(3,4,5)P3: phosphatidylinositol-(3,4,5)-trisphosphate; PtdIns(4,5)P2: phosphatidylinositol-(4,5)-bisphosphate; PTT: photothermal therapies; RAC1: Rac family small GTPase 1; RPS6: ribosomal protein S6; RPS6KB1: ribosomal protein S6 kinase B1; RTKs: receptor tyrosine kinases; SREBF: sterol regulatory element binding transcription factor; TFEB: transcription factor EB; TNBC: triple-negative breast cancer; TSC2: TSC complex subunit 2; ULK1: unc-51 like autophagy activating kinase 1; UPS: ubiquitin-proteasome system.
Keywords: Anti-cancer therapies; MTORC1; MTORC2; macropinocytosis; metabolic reprogramming; methuosis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
Buddleoside alleviates nonalcoholic steatohepatitis by targeting the AMPK-TFEB signaling pathway.Autophagy. 2025 Jun;21(6):1316-1334. doi: 10.1080/15548627.2025.2466145. Epub 2025 Mar 16. Autophagy. 2025. PMID: 39936600
-
Inhibition of lysosomal LAMTOR1 increases autophagy by suppressing the MTORC1 pathway to ameliorate lipid accumulations in MAFLD.Autophagy. 2025 Jul 6:1-17. doi: 10.1080/15548627.2025.2519054. Online ahead of print. Autophagy. 2025. PMID: 40548398
-
An update on cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer.Future Oncol. 2025 Mar;21(6):715-735. doi: 10.1080/14796694.2025.2461443. Epub 2025 Feb 12. Future Oncol. 2025. PMID: 39936282 Review.
-
Emerging dimensions of autophagy in melanoma.Autophagy. 2024 Aug;20(8):1700-1711. doi: 10.1080/15548627.2024.2330261. Epub 2024 Mar 21. Autophagy. 2024. PMID: 38497492 Free PMC article. Review.
-
BIN1 deficiency enhances ULK3-dependent autophagic flux and reduces dendritic size in mouse hippocampal neurons.Autophagy. 2025 Jan;21(1):223-242. doi: 10.1080/15548627.2024.2393932. Epub 2024 Sep 10. Autophagy. 2025. PMID: 39171951
Cited by
-
Macropinocytosis: Both a Target and a Tool for Cancer Therapy.Biomolecules. 2025 Jun 26;15(7):936. doi: 10.3390/biom15070936. Biomolecules. 2025. PMID: 40723808 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
