Weight and Blood-Based Markers of Cachexia Predict Disability, Hospitalization and Worse Survival in Cancer Immunotherapy Patients
- PMID: 39817619
- PMCID: PMC11736629
- DOI: 10.1002/jcsm.13685
Weight and Blood-Based Markers of Cachexia Predict Disability, Hospitalization and Worse Survival in Cancer Immunotherapy Patients
Abstract
Background: Cancer-associated cachexia can inhibit immune checkpoint inhibitor (ICI) therapy efficacy. Cachexia's effect on ICI therapy has not been studied in large cohorts of cancer patients aside from lung cancer. We studied associations between real-world routinely collected clinical cachexia markers and disability-free, hospitalization-free and overall survival of cancer patients.
Methods: A retrospective study was conducted of electronic health records (EHR) of patients with lung, renal cell, melanoma and other cancers treated with ICI therapy at Northwestern Medicine of Chicago, IL, United States, between March 2011 and January 2022. Weight, body mass index, absolute neutrophil and lymphocyte counts, albumin and C-reactive protein (CRP) measures were analysed to calculate the Fearon consensus criteria for cachexia, weight loss grading system (WLGS) score, neutrophil-lymphocyte ratio (NLR), Prognostic Nutritional Index (PNI) and modified Glasgow Prognostic Score (mGPS) at ICI therapy initiation. Kaplan-Meier and Cox proportional hazards analyses were used to determine associations between these metrics and disability-free, hospitalization-free and overall survival.
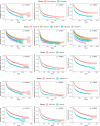
Results: EHR analysis uncovered 3285 cancer patients on ICI therapy (54% > 65 years of age, 50.7% male, 77.7% White). At ICI therapy initiation, 1282 (39.0%) patients had cachexia (consensus criteria), 1641 (50.0%) had a WLGS score ≥ 2, 1806 (55.0%) had an NLR > 3, 1087 (33.1%) had albumin < 3.5 g/dL and 1318 (40.1%) had a PNI < 44. Missing measurements included CRP missing for 98.2% and mGPS missing for 98.6% of patients. Disability-free (n = 1373), hospitalization-free (n = 2374) and overall survival (n = 1599) events were analysed with 1-year rates of 65% (64%-67%), 35% (34%-37%) and 65% (63%-66%), respectively. Multivariate Cox model analyses showed hazard ratios (HR) for cachexia at 1.58 (95% CI 1.38-1.80), 1.47 (95% CI 1.33-1.63) and 1.97 (95% CI 1.75-2.23) for disability, hospitalization and death, respectively. HRs for WLGS ≥ 2 were 1.45 (95% CI 1.28-1.66), 1.37 (95% CI 1.24-1.51) and 1.91 (95% CI 1.69-2.17). HRs for NLR > 3 were 1.57 (95% CI 1.35-1.83), 1.40 (95% CI 1.25-1.58) and 1.95 (95% CI 1.67-2.27). HRs for albumin < 3.5 g/dL were 1.33 (95% CI 1.15-1.54), 1.67 (95% CI 1.50-1.86) and 2.09 (95% CI 1.84-2.36). HRs for PNI < 44 were 1.60 (95% CI 1.39-1.84), 1.46 (95% CI 1.31-1.63) and 2.07 (95% CI 1.80-2.37).
Conclusions: Fearon consensus criteria, WLGS, NLR, albumin and PNI were routinely collected at ICI initiation in regular clinical practice and predictive of worse disability-free, hospitalization-free and overall survival in cancer patients receiving ICI therapy. These routine clinical measures may aid prognostication and decision-making in cancer patients with cachexia.
Keywords: cancer‐associated cachexia; electronic health record data; immune checkpoint inhibitors; survival analysis.
© 2025 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
Dr. Walunas receives unrelated research funding from Gilead Sciences.
Figures
References
-
- Baracos V. E., Martin L., Korc M., Guttridge D. C., and Fearon K. C. H., “Cancer‐Associated Cachexia,” Nature Reviews. Disease Primers 4, no. 1 (2018): 17105. - PubMed
-
- Tisdale M. J., “Cachexia in Cancer Patients,” Nature Reviews Cancer 2, no. 11 (2002): 862–871. - PubMed
-
- Poole R. M., “Pembrolizumab: First Global Approval,” Drugs 74, no. 16 (2014): 1973–1981. - PubMed
-
- Garon E. B., Rizvi N. A., Hui R., et al., “Pembrolizumab for the Treatment of Non–Small‐Cell Lung Cancer,” New England Journal of Medicine 372, no. 21 (2015): 2018–2028. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous