Open-label, phase Ia study of STING agonist BI 1703880 plus ezabenlimab for patients with advanced solid tumors
- PMID: 39817655
- PMCID: PMC11792791
- DOI: 10.1080/14796694.2024.2441107
Open-label, phase Ia study of STING agonist BI 1703880 plus ezabenlimab for patients with advanced solid tumors
Abstract
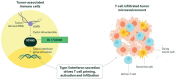
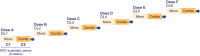
BI 1703880, a novel STimulator of INterferon Genes (STING) agonist, has demonstrated preclinical antitumor activity. As STING activation can upregulate programmed death ligand 1 and human leukocyte antigen in tumor cells, a combination of BI 1703880 and an anti-programmed cell death protein 1-antibody, such as ezabenlimab, may improve efficacy. This first-in-human phase Ia study (NCT05471856) is evaluating BI 1703880 plus ezabenlimab in patients with advanced solid tumors. The study utilizes an innovative lead-in design; all patients receive BI 1703880 monotherapy in Cycle 1 and combination therapy from Cycle 2. The primary endpoint is dose-limiting toxicities during the maximum tolerated dose evaluation period. Results will inform the future development of BI 1703880 for treatment of metastatic or recurrent malignancies.Clinical Trial number: NCT05471856.
Keywords: DNA sensor; PD-1 antibody; STING; STimulator of INterferon Genes; cancer; immune checkpoint inhibitor; immunotherapy; phase I.
Plain language summary
There are many different types of treatments available for patients with cancer. One type of treatment aims to use the patient’s own immune system to target and destroy the cancer, known as immunotherapy. BI 1703880 is a new drug that has been developed to activate the immune system for the treatment of cancer. This study is evaluating BI 1703880 for patients with advanced cancers based on its ability to destroy cancer cells in animal studies. BI 1703880 is being investigated on its own and in combination with another drug, ezabenlimab. The reason for testing these two drugs together is because sometimes cancer cells can become resistant to one type of immunotherapy, making proteins to “turn off” the immune response targeting the cancer. Similar to BI 1703880, ezabenlimab is also an immunotherapy. However, it turns the immune system on in a different way from BI 1703880, which means they may work together to produce a better anticancer result. The objective of the study is to determine a suitable dose of BI 1703880 alone and in combination with ezabenlimab and to see if any side effects occur.
Conflict of interest statement
K Harrington participates in advisory boards/scientific advisory committees for Arch Oncology, AstraZeneca, Bristol Myers Squibb, Boehringer Ingelheim, Codiak, Inzen, Merck-Serono, MSD, Oncolys, Pfizer, Replimune, and Scenic; and has received research funding from AstraZeneca, Boehringer Ingelheim, MSD, and Replimune.
T Doi received research funds from Boehringer Ingelheim, advisory fees from Rakuten Medical, a lecture fee from Ono Pharmaceutical Co., Ltd. and Bristol Myers Squibb, and research expenses from MSD, Taiho Pharmaceutical, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Bristol Myers Squibb, AbbVie, Novartis, Eisai, Janssen Pharmaceutical, Sumitomo Dainippon Pharma, Chugai Pharmaceutical, PRA Health Science, Ono Pharmaceutical Co., Ltd., Eli Lilly, IQVIA, and Merck-Serono.
S Kitano received personal fees from AstraZeneca, Taiho, Novartis, Sumitomo Pharma, Bristol Myers Squibb, Rakuten Medical, GlaxoSmithKline, Merck Biopharma, ImmuniT Research Inc., United Immunity, and PMDA (Pharmaceuticals and Medical Devices Agency); grants and personal fees from Boehringer Ingelheim, Pfizer, MSD, and Eisai; grants from Astellas, Ono Pharmaceutical Co., Ltd., REGENERON, Daiichi Sankyo, Chugai, Takeda, and Eli Lilly Japan K.K.; and grants from Gilead Sciences, PACT Pharma, Takara Bio Inc., Incyte, AMED (Japan Agency for Medical Research and Development), and the JSPS (Japan Society for the Promotion of Science).
EE Parkes has participated in advisory boards for Boehringer Ingelheim, Codiak, and Curadev; and has received research funding from AstraZeneca.
V Gambardella has participated in advisory boards for Boehringer Ingelheim. Research funding: Bayer, Boehringer Ingelheim, and Roche. Institutional funding: Genentech, Merck-Serono, Roche, BeiGene, Bayer, Servier, Eli Lilly, Novartis, Takeda, Astellas, Fibrogen, Amcure, Natera, Sierra Oncology, AstraZeneca, MedImmune, Bristol Myers Squibb, and MSD.
I Moreno participates in advisory boards/scientific advisory committees for Ellipses Pharma, Ltd.
N Fernandez, M Schmohl, and J Barrueco are employees of Boehringer Ingelheim.
P LoRusso has participated in advisory boards for AbbVie, Takeda, Agenus, IQVIA, Pfizer, GlaxoSmithKline, QED Therapeutics, AstraZeneca, EMD Serono, Kyowa Kirin Pharmaceutical Development, Kineta Inc., Zentalis Pharmaceuticals, Molecular Templates, ABL Bio, STCube Pharmaceuticals, I-Mab, Seagen, imCheck, Relay Therapeutics, Stemline (a Menarini company), Compass BADX, Mekanistic, Mersana Therapeutics, BAKX Therapeutics, Scenic Biotech, Qualigen, NeuoTrials, Actuate Therapeutics, Atreca Development, Cullinan, Quanta Therapeutics, Schrodiner, and Boehringer Ingelheim; consultancy for SOTIO, I-Mab, Roivant Sciences; imCORE Alliance for Roche/Genentech; data monitoring committee for Amgen, DrenBio, and SOTIO.
G Alonso declares no conflict of interest.
D Berz reports personal fees from Jazz Pharma, Mirati, EMD, and Sun Pharma.
ME Gutierrez has received consulting fees from Celularity and Guardant.
Amber Wood and Devon Else of Nucleus Global provided writing, editorial support, and formatting assistance, which was contracted and funded by Boehringer Ingelheim. Boehringer Ingelheim was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. This study was supported and funded by Boehringer Ingelheim International GmbH.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). The authors did not receive payment related to the development of the manuscript.
Figures
References
-
- Eggermont AM, Kroemer G, Zitvogel L.. Immunotherapy and the concept of a clinical cure. Eur J Cancer. 2013;49(14):2965–2967. - PubMed
-
- Li S, Luo M, Wang Z, et al. Prolonged activation of innate immune pathways by a polyvalent STING agonist. Nat Biomed Eng. 2021;5(5):455–466. - PMC - PubMed
-
• Proof of concept demonstrating polyvalent STING agonist prolongs the activation of innate-immunity pathways and leads to synergistic therapeutic outcomes in vivo when combined with the STING ligand cyclic GMP–AMP, providing rationale for investigation into STING agonists as an anticancer therapy.
-
- Corrales L, McWhirter SM, Dubensky DW Jr, et al. The host STING pathway at the interface of cancer and immunity. J Clin Invest. 2016;126(7):2404–2411. - PMC - PubMed
-
• Review article outlining the role of the STING pathway in inducing transcription of type I interferon (IFN) genes and the role of type I IFNs in the antitumor immune response, further providing rationale for the investigation of STING agonists as anticancer therapies.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials