Is Kaposi sarcoma a novel comorbidity of cutaneous lymphoma? A systematic review of the literature
- PMID: 39817814
- PMCID: PMC11979566
- DOI: 10.1111/ddg.15625
Is Kaposi sarcoma a novel comorbidity of cutaneous lymphoma? A systematic review of the literature
Abstract
Background and objectives: Patients with cutaneous lymphomas (CL) are at an increased risk of developing secondary malignancies. This study aimed to assess the frequency of association between CL and Kaposi sarcoma (KS) and to identify factors that may promote the co-occurrence of these two diseases.
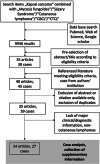
Patients and methods: On January 25, 2024, we conducted a systematic search of four electronic medical databases to identify all published cases of KS associated with CL. The clinical course and outcomes of these patients were summarized. For critical appraisal, we applied the JBI Checklist for Case Reports. The study was registered in the PROSPERO database (CRD42022313204).
Results: A total of 40 articles reporting on 45 patients were assessed for eligibility. We included 27 cases in the final analysis (26 cutaneous T-cell lymphomas, 1 cutaneous B-cell lymphoma). In 71% of cases, the diagnosis of CL preceded KS. Nearly half (48%) of the patients had erythrodermic mycosis fungoides or Sézary syndrome. KS lesions were predominantly limited to the skin, with complete remission achieved in 53% of cases.
Conclusions: The association between KS and CL is rare, limiting our study due to the small sample size and potential reporting bias. Skin-targeted therapies, a restricted T-cell repertoire, and impaired T-cell responses in erythrodermic CTCL patients may contribute to the development of KS.
Keywords: CBCL; CTCL; Kaposi Sarcoma; Mycosis fungoides; Sézary Syndrome; cutaneous lymphoma.
© 2025 The Author(s). Journal der Deutschen Dermatologischen Gesellschaft published by John Wiley & Sons Ltd on behalf of Deutsche Dermatologische Gesellschaft.
Conflict of interest statement
R.K.C.M. has received consultant, speaker fees, and travel support from Kyowa Kirin GmbH, Recordati Rare Diseases, and Takeda Pharmaceutical and has served as a principal investigator in clinical trials conducted by Innate Pharma and 4SC GmbH. J.H. has received speaker fees and travel grants from Bristol Myers Squibb (BMS) and Kyowa Kirin. G.P. has received consultant, speaker fees, or travel grants from BMS, MSD, Novartis, Amgen, and Sun Pharma. R.S. has received research grants, scientific awards, or honoraria for participation in advisory boards, clinical trials, or as a speaker for the following organizations: AbbVie Inc., AbbVie Deutschland GmbH & Co. KG, Almirall Hermal GmbH, Amgen GmbH, Bayer Schering Pharma AG, Boehringer Ingelheim Pharma GmbH & Co. KG, Bruno Bloch Stiftung, Celgene/Bristol Myers Squibb, CSL Behring, Charité Research Organisation GmbH, Flexopharm GmbH & Co. KG, ICON plc, IQVIA RDS GmbH, Incyte Corporation, Janssen‐Cilag GmbH, MoonLake Immunotherapeutics AG, Novartis Pharma GmbH, Parexel International GmbH, Rheinische Friedrich‐Wilhelms‐Universität Bonn, Sanofi‐Aventis Deutschland GmbH, TFS GmbH, UCB Biopharma SPRL, Universitätsmedizin Greifswald, and Wundnetz Berlin‐Brandenburg e.V. M.S. has received consultant, speaker fees, and travel grants from BMS, MSD, Roche, Kyowa Kirin, Novartis, Sanofi Genzyme, Pierre Fabre, Sun Pharma, and Immunocore. G.D. has received consultant, speaker fees, and travel support from Kyowa Kirin, Recordati Rare Diseases, Helsinn, Mallinckrodt Therakos, and Takeda Pharmaceutical.
Figures
Similar articles
-
Interventions for mycosis fungoides.Cochrane Database Syst Rev. 2012 Sep 12;(9):CD008946. doi: 10.1002/14651858.CD008946.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2020 Jul 7;7:CD008946. doi: 10.1002/14651858.CD008946.pub3. PMID: 22972128 Updated.
-
Chimeric antigen receptor (CAR) T-cell therapy for people with relapsed or refractory diffuse large B-cell lymphoma.Cochrane Database Syst Rev. 2021 Sep 13;9(9):CD013365. doi: 10.1002/14651858.CD013365.pub2. Cochrane Database Syst Rev. 2021. PMID: 34515338 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Interventions for Old World cutaneous leishmaniasis.Cochrane Database Syst Rev. 2017 Dec 1;12(12):CD005067. doi: 10.1002/14651858.CD005067.pub5. Cochrane Database Syst Rev. 2017. PMID: 29192424 Free PMC article.
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
-
- Denis D, Seta V, Regnier‐Rosencher E, et al. A fifth subtype of Kaposi's sarcoma, classic Kaposi's sarcoma in men who have sex with men: a cohort study in Paris. J Eur Acad Dermatol Venereol. 2018;32:1377‐1384. - PubMed
-
- Esser S, Schöfer H, Hoffmann C, Claßen J. S1 Guidelines for the Kaposi Sarcoma. J Dtsch Dermatol Ges. 2022;20:892‐904. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical