Isogenic Transplantation of Hybrid Artificial Pleural Tissue Consisting of Rat Cells and Polyglycolic Acid Nanofiber Sheet Induces Restoration of Mesothelial Defects in Rat Model
- PMID: 39817871
- PMCID: PMC12019103
- DOI: 10.1111/aor.14947
Isogenic Transplantation of Hybrid Artificial Pleural Tissue Consisting of Rat Cells and Polyglycolic Acid Nanofiber Sheet Induces Restoration of Mesothelial Defects in Rat Model
Abstract
Background: Impairment of the visceral pleura following thoracic surgery often leads to air leaks and intrathoracic adhesions. For preventing such complications, mesothelial cell proliferation at the pleural defects can be effective. To develop new materials for pleural defects restoration, we constructed a hybrid artificial pleural tissue (H-APLT) combining polyglycolic acid (PGA) nanofiber sheets with a three-dimensional culture of mesothelial cells and fibroblasts and evaluated its therapeutic efficacy in a rat pleural defect model.
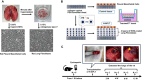
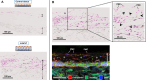
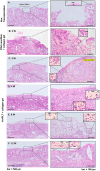
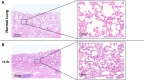
Methods: After rat lungs were harvested, pleural mesothelial cells and lung fibroblasts were cultured separately. To construct H-APLT, the cells were then coated with multiple layers of fibronectin and gelatin, followed by a single layer of mesothelial cells on top of multiple layers of fibroblasts accumulated onto a collagen-coated PGA nanofiber sheet. Left lateral thoracotomy was performed, and H-APLTs were transplanted into a rat model with pleural defects (N = 8). After 2-12 weeks of transplantation, lung resection and histological analyses were performed.
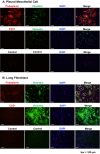
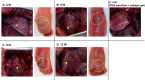
Results: H-APLTs exhibited a pleural structure with a highly integrated mesothelial layer in vitro. After transplantation, all eight rats survived until sacrifice. At 12 weeks post-transplantation, the mesothelial layer on the lung surface was observed to be without defects with no intrathoracic adhesions detected.
Conclusion: Successful isogenic engraftment of H-APLTs was achieved in a rat model of pleural defects. The combination of accumulated fibroblasts and collagen-coated PGA nanofiber sheets contributed to the maintenance of the mesothelial layer's structure and function, potentially preventing air leaks and intrathoracic adhesions.
Keywords: artificial pleural tissue; cell‐accumulation method; mesothelial cells; polyglycolic acid nanofiber sheet; tissue engineering.
© 2025 The Author(s). Artificial Organs published by International Center for Artificial Organ and Transplantation (ICAOT) and Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Mutsaers S. E., Prele C. M., Brody A. R., and Idell S., “Pathogenesis of Pleural Fibrosis,” Respirology 9, no. 4 (2004): 428–440. - PubMed
-
- Mutsaers S. E., Prêle C. M., Pengelly S., and Herrick S. E., “Mesothelial Cells and Peritoneal Homeostasis,” Fertility and Sterility 106, no. 5 (2016): 1018–1024. - PubMed
-
- Mutsaers S. E., “Mesothelial Cells: Their Structure, Function and Role in Serosal Repair,” Respirology 7, no. 3 (2002): 171–191. - PubMed
-
- Attaar A., Luketich J. D., Schuchert M. J., Winger D. G., Sarkaria I. S., and Nason K. S., “Prolonged Air Leak After Pulmonary Resection Increases Risk of Noncardiac Complications, Readmission, and Delayed Hospital Discharge: A Propensity Score‐Adjusted Analysis,” Annals of Surgery 273, no. 1 (2021): 163–172. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

