Two-Step Acoustic Cell Separation Based on Cell Size and Acoustic Impedance─toward Isolation of Viable Circulating Tumor Cells
- PMID: 39818757
- PMCID: PMC11800186
- DOI: 10.1021/acs.analchem.4c04911
Two-Step Acoustic Cell Separation Based on Cell Size and Acoustic Impedance─toward Isolation of Viable Circulating Tumor Cells
Abstract
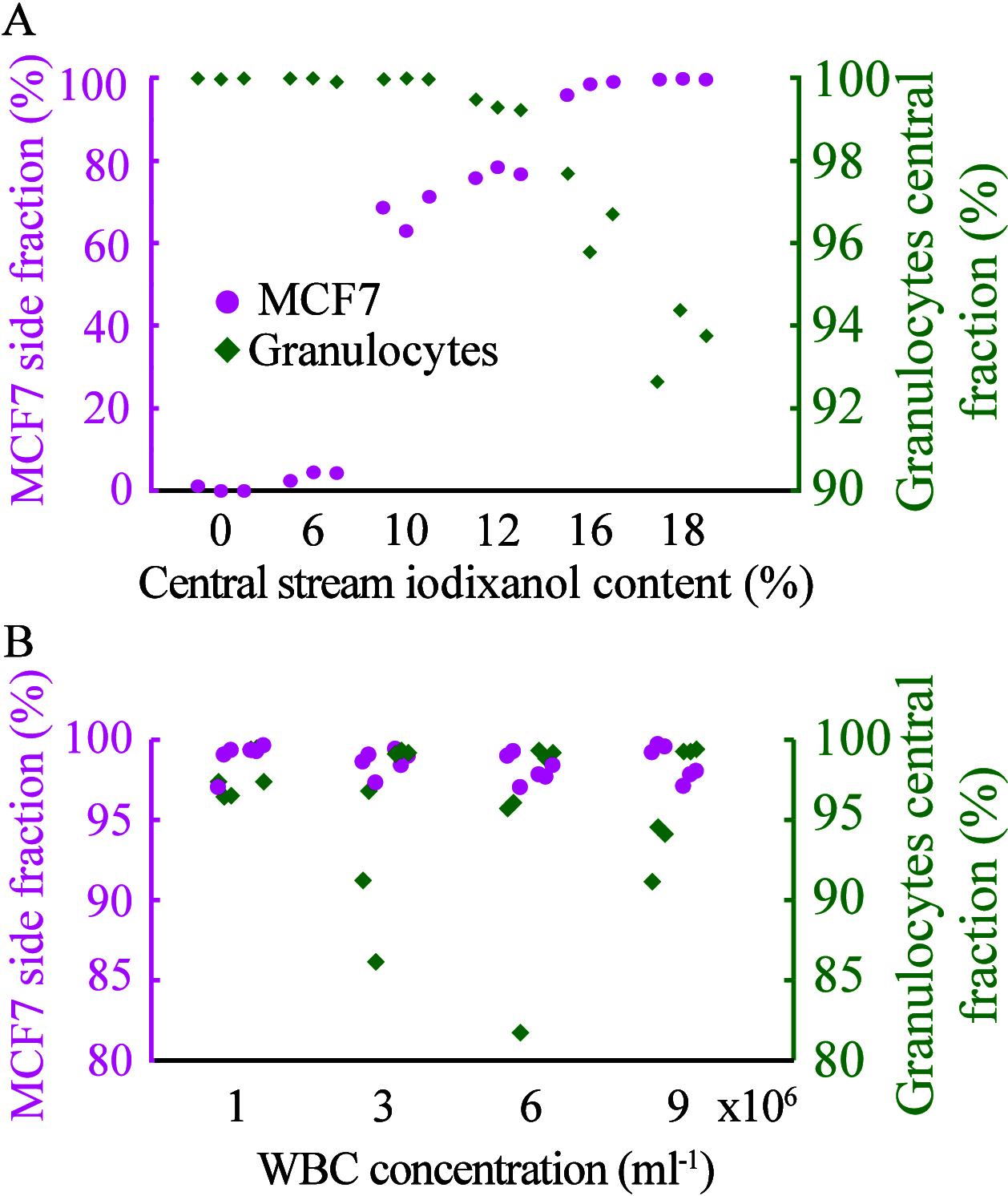
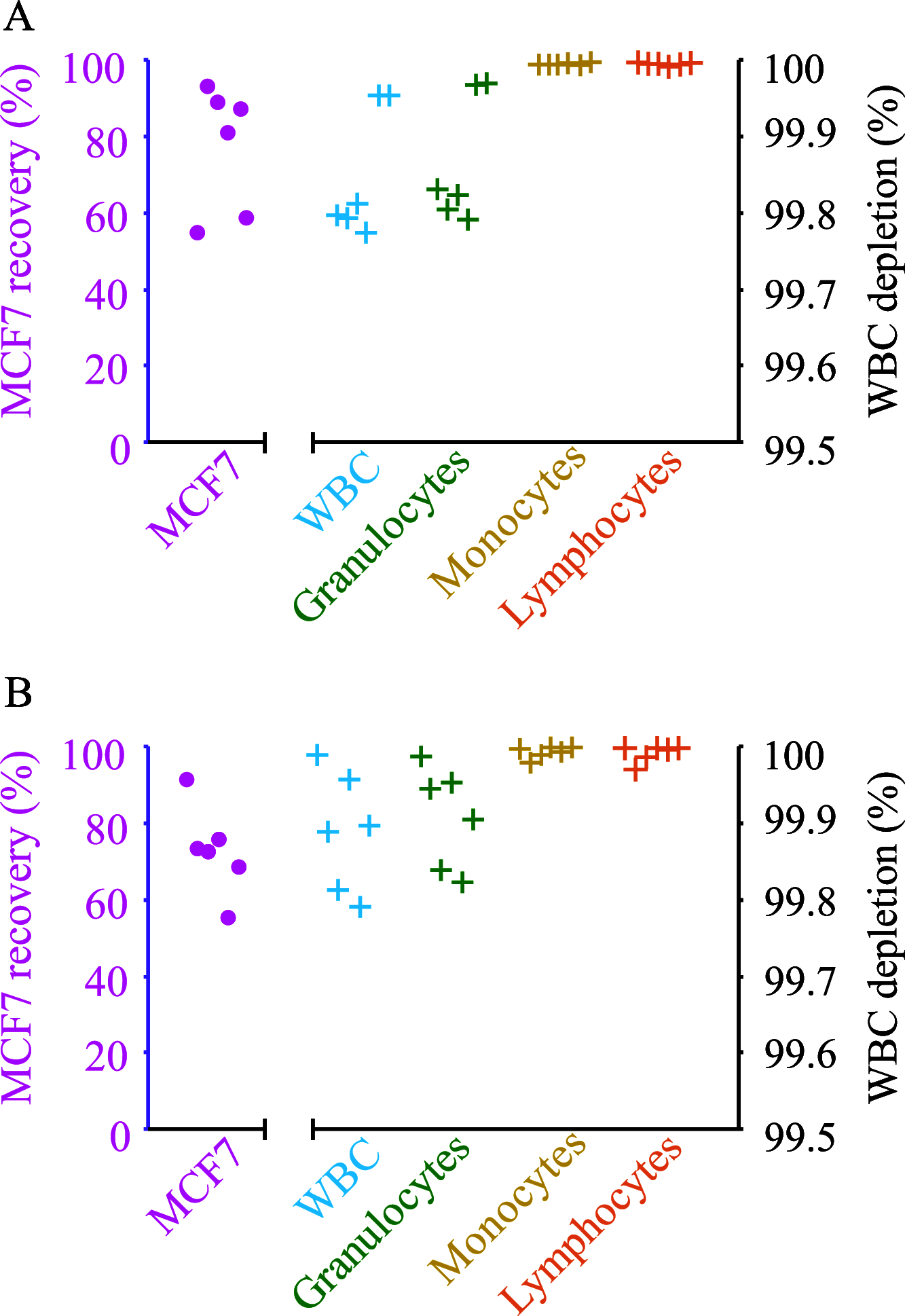
Isolation and characterization of circulating tumor cells (CTCs) present a noninvasive alternative to monitor disease progression in individual patients. However, the heterogeneous lineage specificity of CTCs makes it difficult to isolate and identify possible CTCs by a liquid biopsy. Better label-free methods for the isolation of viable CTCs are needed. Our solution is a combined approach that is inherently epitope independent. Cells are separated by size-sensitive acoustophoresis using an ultrasonic standing wave field, followed by size-insensitive, acoustic barrier-medium focusing, which enables the enrichment of viable cancer cells in blood. With standard acoustophoresis in homogeneous medium, lymphocytes and monocytes were efficiently removed, while removal of granulocytes from the target MCF7 breast cancer cells was not possible due to overlapping acoustic migration velocities for viable cells. Remaining granulocytes were removed by a second separation step with an acoustic impedance barrier-medium selectively blocking the transport of MCF7 cells to generate a clean cancer cell fraction. For two series of 500 mL samples containing 5 × 105 white blood cells, spiked with 2 × 104 or 1 × 103 MCF7 cells, the recovery of MCF7 cells was 77.3% with a 99.9% depletion of white blood cells in the final cancer cell fraction. The most abundant contaminating cell type was granulocytes (85.9% of remaining cells). Nearly all lymphocytes (99.996%) and monocytes (99.995%) were depleted. A two-step acoustic cell separation based on cell size and acoustic impedance is well suited to generate a purified cancer cell fraction as a preparatory step for downstream single-cell analysis.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Acoustic Enrichment of Heterogeneous Circulating Tumor Cells and Clusters from Metastatic Prostate Cancer Patients.Anal Chem. 2024 May 7;96(18):6914-6921. doi: 10.1021/acs.analchem.3c05371. Epub 2024 Apr 24. Anal Chem. 2024. PMID: 38655666 Free PMC article.
-
Acoustic impedance-based size-independent isolation of circulating tumour cells from blood using acoustophoresis.Lab Chip. 2018 Dec 4;18(24):3802-3813. doi: 10.1039/c8lc00921j. Lab Chip. 2018. PMID: 30402651
-
Clinical-Scale Cell-Surface-Marker Independent Acoustic Microfluidic Enrichment of Tumor Cells from Blood.Anal Chem. 2017 Nov 21;89(22):11954-11961. doi: 10.1021/acs.analchem.7b01458. Epub 2017 Nov 9. Anal Chem. 2017. PMID: 29087172 Free PMC article.
-
[Recent advances in isolation and detection of circulating tumor cells with a microfluidic system].Se Pu. 2022 Mar 8;40(3):213-223. doi: 10.3724/SP.J.1123.2021.07009. Se Pu. 2022. PMID: 35243831 Free PMC article. Review. Chinese.
-
Size-based separation methods of circulating tumor cells.Adv Drug Deliv Rev. 2018 Feb 1;125:3-20. doi: 10.1016/j.addr.2018.01.002. Epub 2018 Jan 8. Adv Drug Deliv Rev. 2018. PMID: 29326054 Review.
References
-
- de Bono J. S.; Scher H. I.; Montgomery R. B.; Parker C.; Miller M. C.; Tissing H.; Doyle G. V.; Terstappen L. W. W. M.; Pienta K. J.; Raghavan D. Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2008, 14, 6302–6309. 10.1158/1078-0432.CCR-08-0872. - DOI - PubMed
-
- Cohen S. J.; Punt C. J. A.; Iannotti N.; Saidman B. H.; Sabbath K. D.; Gabrail N. Y.; Picus J.; Morse M.; Mitchell E.; Miller M. C.; et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin Oncol 2008, 26, 3213–3221. 10.1200/JCO.2007.15.8923. - DOI - PubMed
-
- Cristofanilli M.; Budd G. T.; Ellis M. J.; Stopeck A.; Matera J.; Miller M. C.; Reuben J. M.; Doyle G. V.; Allard W. J.; Terstappen L. W. M. M.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. New Engl J. Med. 2004, 351, 781–791. 10.1056/NEJMoa040766. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

