Discovery of (3-Phenylcarbamoyl-3,4-dihydro-2 H-pyrrol-2-yl)phosphonates as Imidazoline I2 Receptor Ligands with Anti-Alzheimer and Analgesic Properties
- PMID: 39818939
- PMCID: PMC11831594
- DOI: 10.1021/acs.jmedchem.4c01644
Discovery of (3-Phenylcarbamoyl-3,4-dihydro-2 H-pyrrol-2-yl)phosphonates as Imidazoline I2 Receptor Ligands with Anti-Alzheimer and Analgesic Properties
Abstract
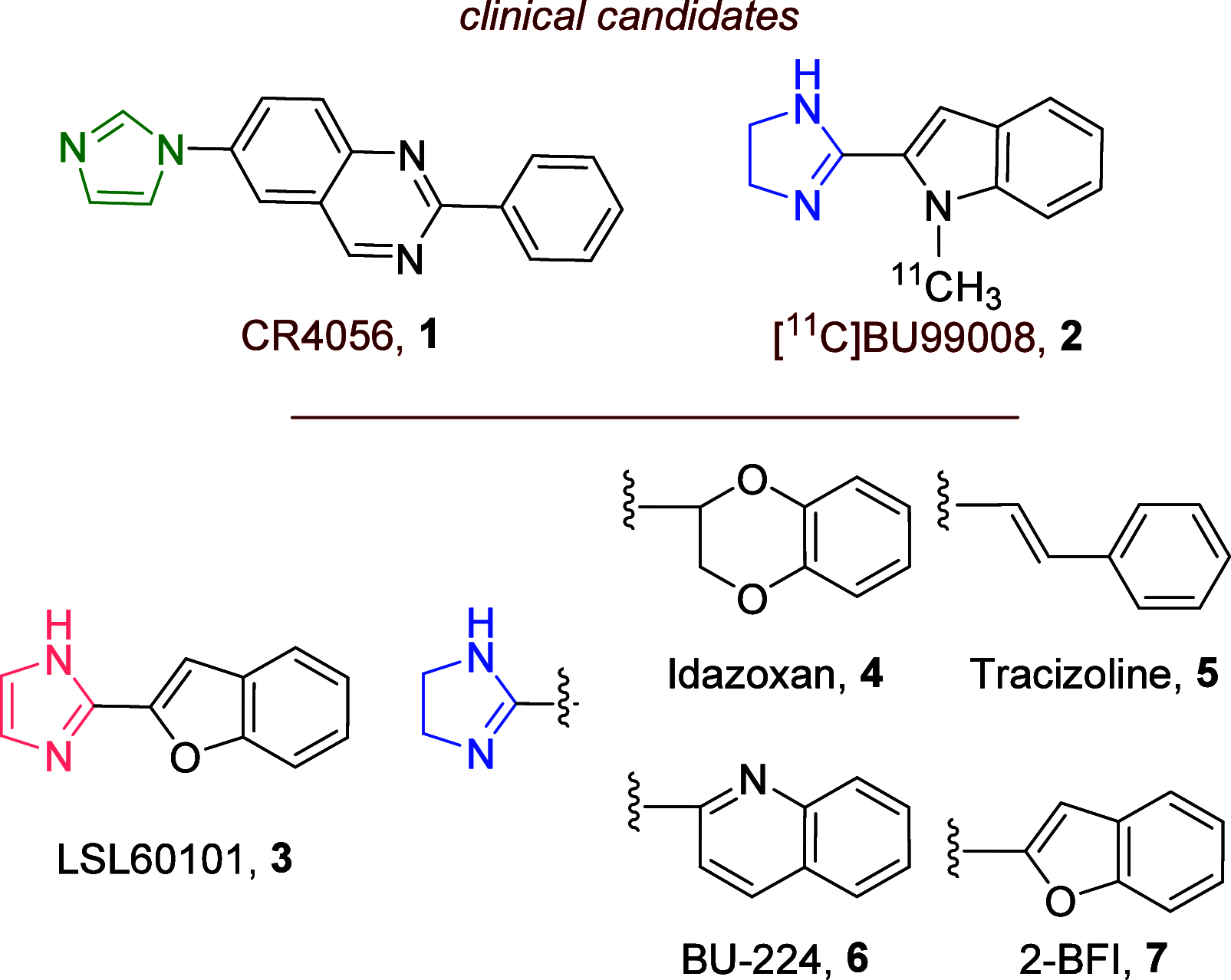
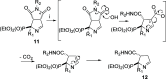
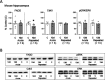
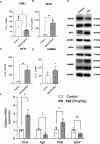
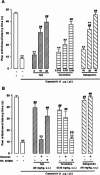
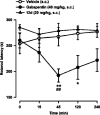
Imidazoline I2 receptors (I2-IRs) are altered in Alzheimer's disease (AD) patients and are associated with analgesia. I2-IRs are not structurally described, and their pharmacological characterization relies on their modulation by highly affine ligands. Herein, we describe the synthesis of (3-phenylcarbamoyl-3,4-dihydro-2H-pyrrol-2-yl)phosphonates endowed with relevant affinities for I2-IRs in human brain tissues. The optimal ADME and pharmacokinetic profile of a selected compound, 12d, secured its in vivo exploration in a senescence accelerated prone 8 mice revealing improvement in the cognitive impairment and unveiling the mechanism of action by analyzing specific AD biomarkers. The treatment of a capsaicin-induced mechanical hypersensitivity murine model with 12d revealed analgesic properties devoid of motor coordination issues. The target engagement of 12d was demonstrated by suppression of the analgesic effect by pretreatment with idazoxan. Overall, 12d is a putative candidate for advancing preclinical phases and supports the modulation of I2-IRs as an innovative approach for therapeutics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
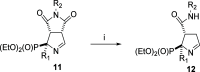


References
-
- Bousquet P.; Feldman J.; Schwartz J. Central Cardiovascular Effects of Alpha Adrenergic Drugs: Differences between Catecholamines and Imidazolines. J. Pharmacol. Exp. Ther. 1984, 230 (1), 232–236. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

