Alterations in Gut Microbiome-Host Relationships After Immune Perturbation in Patients With Multiple Sclerosis
- PMID: 39819054
- PMCID: PMC11741292
- DOI: 10.1212/NXI.0000000000200355
Alterations in Gut Microbiome-Host Relationships After Immune Perturbation in Patients With Multiple Sclerosis
Abstract
Background and objectives: Gut microbial symbionts have been shown to influence the development of autoimmunity in multiple sclerosis (MS). Emerging research points to an important relationship between the microbial-IgA interface and MS pathophysiology. IgA-secreting B cells are observed in the MS brain, and shifts in gut bacteria-IgA binding have been described in some patients with MS. However, the relationships between the gut microbiome and the host immune response, particularly regarding B-cell-depleting immunomodulation, remain underexplored. This study aimed to evaluate the composition of the gut microbiome in patients with newly diagnosed MS at baseline and after B-cell depletion, using long-read sequencing for enhanced taxonomic resolution. We further aimed to investigate the host/microbiome interface by evaluating microbe/immunoglobulin A relationships.
Methods: We collected stool samples from 43 patients with newly diagnosed, untreated MS and 42 matched healthy controls. Nineteen patients with MS initiated anti-CD20 monoclonal antibody treatment and donated additional stool samples after 6 months of treatment. We evaluated the host-microbial interface using bacterial flow cytometry and long-read 16S rRNA gene amplicon sequencing. We used Immune Coating Scores to compare the proportions of bacteria identified in the IgA-coated vs IgA-uncoated bacterial fractions.
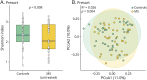
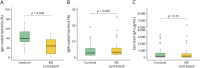
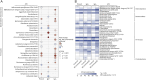
Results: Patients with untreated, newly diagnosed MS showed significant reductions in IgA-bound fecal microbiota compared with controls. Using multiple linear regression models adjusted for potential confounders, we observed significant (p < 0.05) changes in the abundance and prevalence of various strain-level gut bacteria amplicon sequence variants (ASVs) within both total and IgA-coated bacterial fractions. Some changes (e.g., decreased relative abundance of a Faecalibacterium prausnitzii variant in MS) were consistent with previous reports, while others (e.g., increased relative abundance and prevalence of Monoglobus pectinyliticus in MS) were novel. Immune Coating Scores identified subsets of organisms for which normal IgA-coating patterns were disrupted at the onset of MS, as well as those (particularly Akkermansia muciniphila) whose IgA-coating became more aligned with controls after therapy.
Discussion: This analysis of gut microbial ASVs reveals shifts in taxonomic strains induced by immune modulation in MS.
Conflict of interest statement
Among our authors, in the last 2 years, E.E. Longbrake has received honoraria for consulting from Bristol Myers Squibb, EMD Serono, Genentech, Novartis, and Genzyme and research support from Biogen, LabCorp, Intus, and Genentech. She is an associate editor for Annals of Neurology. D.A. Hafler has received research funding from Bristol-Myers Squibb, Novartis, Sanofi, and Genentech. He has been a consultant for Bayer Pharmaceuticals, Repertoire Inc., Bristol Myers Squibb, Compass Therapeutics, EMD Serono, Genentech, Juno therapeutics, Novartis Pharmaceuticals, Proclara Biosciences, Sage Therapeutics, and Sanofi Genzyme. The other authors report no disclosures. All other authors report no relevant disclosures. Go to
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
