Paramagnetic rim lesion formation is predicted by the initial gadolinium-enhancing lesion diameter
- PMID: 39819146
- PMCID: PMC11919566
- DOI: 10.1177/13524585241310764
Paramagnetic rim lesion formation is predicted by the initial gadolinium-enhancing lesion diameter
Abstract
Background: Paramagnetic rim lesions (PRLs) are a magnetic resonance imaging (MRI) marker of compartmentalized intraparenchymal inflammation.
Objectives: The primary objective was to investigate clinical, demographic, and MRI factors that may be predictive of the future formation of PRL.
Methods: This is a retrospective analysis of longitudinal data. Patients were included if they had ⩾1 gadolinium-enhancing lesion on any historical MRI and follow-up scan(s) ⩾6 months afterward on a standardized 3T MRI using the filtered phase component of a susceptibility-sensitive sequence ("SWAN"). Regression and machine-learning models were used to identify the predictive ability of demographic, clinical, immunological, treatment-related, and MRI predictors of PRL formation.
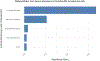
Results: A total of 64 patients having 229 contrast-enhancing lesions (CELs) were included. Among all predictors, the diameter of the initial enhancing lesion was the most influential for determining subsequent PRL formation; every millimeter increase in diameter increased the risk of PRL formation by 44%. Other factors did not contribute additional information; the administration of steroids was not associated with any effect.
Conclusions: The long-axis diameter of a CEL is the best translational predictor of subsequent PRL formation at follow-up. This measure holds promise as a method to identify patients at high risk of chronic active lesion formation during the acute inflammatory window.
Keywords: MRI; biomarkers; multiple sclerosis; paramagnetic rim lesion; steroids glucocorticoids.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare no financial conflicts of interest regarding material in this study.M. Al Gburi reports no disclosures.M. Mazzola reports no disclosures.M. Absinta received research support from the Conrad N. Hilton Foundation (17313), the Cariplo Foundation (2019-1677), the Roche Foundation, the FRRB Early Career Award (1750327), the National MS Society (NMSS, FG2093-A-1 and RFA2203-39325), the International Progressive MS Alliance (PA-2107-38081), and the Fondazione Italiana Sclerosi Multipla (FISM, 2023/R-Single/015 co-financed with the “5 per mille” public funding). She received consulting honoraria from Sanofi, GSK, Biogen, Immunic Therapeutics, and Abata Therapeutics, not directly related to the present study.M. I. Gaitán reports no disclosures.D. S. Reich is funded by the Intramural Research Program of NINDS, and has received research support from Abata and Sanofi for PRL-related research, not directly related to the present study.S. K. Dundamadappa reports no disclosures.C. C. Hemond received research support for this project from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number K23NS126718. He has previously received consulting honoraria from VIVIO health, unrelated to the current study.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

