Epidemiology of transthyretin (ATTR) amyloidosis: a systematic literature review
- PMID: 39819351
- PMCID: PMC11740649
- DOI: 10.1186/s13023-025-03547-0
Epidemiology of transthyretin (ATTR) amyloidosis: a systematic literature review
Abstract
Introduction: Significant advances in the treatment of transthyretin (ATTR) amyloidosis has led to an evolving understanding of the epidemiology of this condition. This systematic literature review (SLR) aims to synthesize current evidence on epidemiology and mortality outcomes in ATTR amyloidosis, addressing the need for a comprehensive understanding of its current global impact.
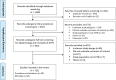
Methods: An SLR of the literature from January 2018 to April 2023 was conducted using the Medline and Embase databases. The review followed the PRISMA guidelines. Studies evaluating populations with genotypes and phenotypes of ATTR amyloidosis (variant and wild-type cardiomyopathy, polyneuropathy, and mixed) were included. Observational studies, systematic reviews, and meta-analyses were eligible, while reports, commentaries, clinical trials, and non-ATTR amyloidosis studies were excluded. Extracted data included prevalence, incidence, and mortality rates.
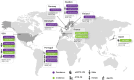
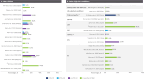
Results: Of the 1,458 studies identified, 113 met the inclusion criteria. Forty-nine studies reported on epidemiology, while 64 focused on mortality rates in cohorts of patients with ATTR amyloidosis from Europe (n = 16), North America (n = 26), Asia (n = 5), and Australia (n = 2). No studies were found that exclusively focused on ATTR amyloidosis in Africa or South America. ATTR prevalence ranged from 6.1/million in the US to 232/million in Portugal with very limited data on ATTR-PN. The 2-year mortality risk ranged from 10 to 30% among wild-type ATTR-CM and from 10 to 50% for variant type of ATTR-CM.
Conclusions: This SLR demonstrated heterogeneity in ATTR epidemiology and mortality rates across global regions. Further investigation is needed to address knowledge gaps of the epidemiology and burden of ATTR, which may improve early diagnosis and management.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: FD, FM and DS are full-time employees of Evidera. DD received consulting fees and speakers’ honoraria from Akcea, Alnylam, Pfizer and AstraZeneca. NS, EW, and KJ are full-time employees of AstraZeneca and hold stock in the company.
Figures
References
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

