Unraveling calcium dysregulation and autoimmunity in immune mediated rippling muscle disease
- PMID: 39819455
- PMCID: PMC11736958
- DOI: 10.1186/s40478-025-01926-z
Unraveling calcium dysregulation and autoimmunity in immune mediated rippling muscle disease
Erratum in
-
Correction: Unraveling calcium dysregulation and autoimmunity in immune mediated rippling muscle disease.Acta Neuropathol Commun. 2025 Mar 24;13(1):66. doi: 10.1186/s40478-025-01981-6. Acta Neuropathol Commun. 2025. PMID: 40128859 Free PMC article. No abstract available.
Abstract
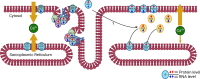
Rippling Muscle Disease (RMD) is a rare skeletal myopathy characterized by abnormal muscular excitability manifesting with wave-like muscle contractions and percussion-induced muscle mounding. Hereditary RMD is associated with caveolin-3 or cavin-1 mutations. Recently, we identified cavin 4 autoantibodies as a biomarker of immune-mediated RMD (iRMD), though the underlying disease-mechanisms remain poorly understood. Transcriptomic studies were performed on muscle biopsies of 8 patients (5 males; 3 females; ages 26-to-80) with iRMD. Subsequent pathway analysis compared iRMD to human non-disease control and disease control (dermatomyositis) muscle samples. Transcriptomic studies demonstrated changes in key pathways of muscle contraction and development. All iRMD samples had significantly upregulated cavin-4 expression compared to controls, likely compensatory for autoantibody-mediated protein degradation. Proteins involved in muscle relaxation (including SERCA1, PMCA and PLN) were significantly increased in iRMD compared to controls. Comparison of iRMD to dermatomyositis transcriptomics demonstrated significant overlap in immune pathways, and the IL-6 signaling pathway was markedly increased in all iRMD patient muscle biopsies and increased in the majority of iRMD patients' serum. This study represents the first muscle transcriptomic analysis of iRMD patients and dissects underlying disease mechanisms. Increase of sarcolemmal and cellular calcium channels as well as PLN, an inhibitor of the SERCA pump for calcium into the sarcoplasm, likely alters the calcium dynamics in iRMD. These changes in crucial components of muscle relaxation may underlie rippling by altering calcium flux. Our findings provide crucial insights into the differential expression of genes regulating muscle relaxation and highlight potential disease pathomechanisms.
Keywords: Immune mediated rippling muscle disease; Interferon; Interleukin-6; Myopathy; Transcriptomics.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Mayo Clinic Institutional Review Board (IRB#18-010637). Consent for publication: All authors have reviewed and approved the final version of the manuscript and consent to its publication in Acta Neuropathologica Communications. The study was approved by the Mayo Clinic Institutional Review Board (IRB#18-010637). Competing interests: MM has received personal compensation for serving on a Scientific Advisory or Data Safety Monitoring board for Argenx on an unrelated topic and for serving as an Associate Editor for Neurology Genetics, AAN. SP has received personal compensation for consulting roles with Genentech, Sage Therapeutics, Prime Therapeutics, UCB, Roche/Genentech, and Arialys Therapeutics, as well as for serving on a Scientific Advisory or Data Safety Monitoring board for UCB, Inc., Genentech, and F. Hoffman/LaRoche, for advisory work with Hoffman/LaRoche AG and Alexion, for consulting roles with Astellas, Alexion, MedImmune/Viela Bio, and Roche/Genentech. His institution has received research support from Grifols, NIH, Viela Bio/MedImmune/Horizon, Alexion Pharmaceuticals, F. Hoffman/LaRoche/Genentech, NovelMed, and AstraZeneca. SP holds intellectual property interests in healthcare-related discoveries and technologies. D.D. has consulted for UCB, Immunovant, Argenx, Arialys and Astellas pharmaceuticals. All compensation for consulting activities is paid directly to Mayo Clinic. He is a named inventor on filed patent that relates to KLHL11 as marker of autoimmunity and germ cell tumor. He has patents pending for LUZP4-IgG, cavin-4-IgG and SKOR2 IgG as markers of neurological autoimmunity. He has received funding from the DOD (CA210208 & PR220430), David J. Tomassoni ALS Research Grant Program and UCB.
Figures






References
-
- Schulte-Mattler WJ et al (2005) Immune-mediated rippling muscle disease. Neurology 64(2):364–367 - PubMed
-
- McNally EM et al (1998) Caveolin-3 in muscular dystrophy. Hum Mol Genet 7(5):871–877 - PubMed
-
- Muller JS et al (2006) Novel splice site mutation in the caveolin-3 gene leading to autosomal recessive limb girdle muscular dystrophy. Neuromuscul Disord 16(7):432–436 - PubMed
-
- Berling E et al (2023) Caveolinopathy: Clinical, histological, and muscle imaging features and follow-up in a multicenter retrospective cohort. Eur J Neurol 30(8):2506–2517 - PubMed
-
- Fulizio L et al (2005) Molecular and muscle pathology in a series of caveolinopathy patients. Hum Mutat 25(1):82–89 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

