Tissue-specific roles of de novo DNA methyltransferases
- PMID: 39819598
- PMCID: PMC11740433
- DOI: 10.1186/s13072-024-00566-2
Tissue-specific roles of de novo DNA methyltransferases
Abstract
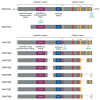
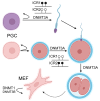
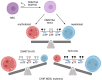
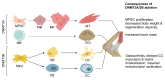
DNA methylation, catalyzed by DNA methyltransferases (DNMT), plays pivotal role in regulating embryonic development, gene expression, adaption to environmental stress, and maintaining genome integrity. DNMT family consists of DNMT1, DNMT3A, DNMT3B, and the enzymatically inactive DNMT3L. DNMT3A and DNMT3B establish novel methylation patterns maintained by DNMT1 during replication. Genetic variants of DNMT3A and DNMT3B cause rare diseases such as Tatton-Brown-Rahman and ICF syndromes. Additionally, somatic mutations cause common conditions such as osteoarthritis, osteoporosis, clonal hematopoiesis of indeterminate potential (CHIP), hematologic malignancies, and cancer. While DNMTs have been extensively studied in vitro, in early development and in disease, their detailed physiologic roles remain less understood as in vivo investigations are hindered by the embryonic or perinatal lethality of the knockout mice. To circumvent this problem, tissue-specific Dnmt3a and Dnmt3b knockouts were engineered. This review explores their diverse molecular roles across various organs and cell types and characterizes the phenotype of the knockout mice. We provide a comprehensive collection of over forty tissue-specific knockout models generated by cre recombinase. We highlight the distinct functions of DNMT3A and DNMT3B in germ cells, early development, uterus, hematopoietic differentiation, musculoskeletal development, visceral organs, and nervous system. Our findings indicate that DNMT3A primarily regulates hematopoietic differentiation, while DNMT3B is crucial for cartilage homeostasis and ossification. We emphasize the context-dependent roles of DNMT3A and DNMT3B and demonstrate that they also complement DNMT1 maintenance methyltransferase activity. Overall, the expression patterns of DNMTs across tissues provide insights into potential therapeutic applications for treating neurologic diseases, cancer, and osteoporosis.
Keywords: De novo methyltransferase; Cre recombinase; DNA methylation; Development; Differentiation; Dnmt3a; Dnmt3b; Knockout; LoxP; Stem cells; Tissue-specific.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: TA is Associate Editor of Epigenetics & Chromatin. The other authors declare no competing interests.
Figures

References
-
- Greenberg MVC, Bourc’his D. The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol. 2019;20(10):590–607. - PubMed
-
- Arányi T, Kerjean A, Tóth S, Mallet J, Meloni R, Páldi A. Paradoxical methylation of the tyrosine hydroxylase gene in mouse preimplantation embryos. Genomics. 2002;80(6):558–63. - PubMed
-
- Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14(3):204–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

