Dysgeusia in MASLD-related advanced chronic liver disease (ACLD): a silent driver towards the "Bermuda" triangle of malnutrition-sarcopenia-frailty severely affecting prognosis
- PMID: 39819605
- PMCID: PMC11736961
- DOI: 10.1186/s12937-025-01074-z
Dysgeusia in MASLD-related advanced chronic liver disease (ACLD): a silent driver towards the "Bermuda" triangle of malnutrition-sarcopenia-frailty severely affecting prognosis
Abstract
Background: Dysgeusia is a distortion of the sense of taste whose prevalence and relationship with nutritional status in Metabolic dysfunction-associated Steatotic Liver Disease (MASLD)-related advanced chronic liver disease (ACLD) have never been systematically explored.
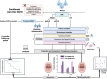
Methods: 200 MASLD patients [60 ≤ F3 fibrosis, 70 compensated ACLD (cACLD), and 70 decompensated (dACLD)] were enrolled. At baseline, the Child-Pugh (CP) score was determined. Dietary habits, body composition, and frailty were evaluated. The European Working Group (EWGSOP2) criteria defined sarcopenia. Dysgeusia was assessed by the Dysgeusia-Total-Score (DTS). A visual analog scale identified appetite impairment (VASAI). During a 6-month follow-up, liver-related decompensation events (LRDEs) were recorded.
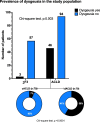
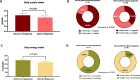
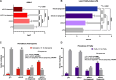
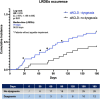
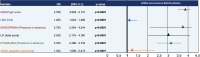
Results: The prevalence of dysgeusia increased with the liver disease progression, appearing significantly higher in ACLD compared with ≤ F3 (65.7% vs 5%, p:0.003), as well as in dACLD compared to cACLD patients (58.5 vs 7.1% p < 0.0001). On 41 dACLD patients presenting dysgeusia, 37 (90.2%) showed a significant impairment of appetite levels. In dACLD, the CP score was positively correlated with both DTS (R:0.742) and VASAI (R:0.704), as well as DTS was directly correlated with VASAI (R:0.765) (all p < 0.0001). Compared with dACLD patients without dysgeusia, dysgeusia-affected dACLD patients presented a lower daily protein intake (g/kg/die) (1.55 ± 0.192 vs 1.34 ± 0.15, p < 0.0001). Sarcopenia (70.7 vs 41.3%) and frailty (69.29 vs 37.9%) were significantly more prevalent in dysgeusia-affected dACLD individuals (both p < 0.0001). These patients showed a higher risk of LRDEs occurrence during the follow-up [HR:2.205; C.I. 95%:1.186-4.099; p:0.01]. Logistic regression analysis revealed dysgeusia (aOR: 3.32), appetite impairment (aOR:1.32), sarcopenia (aOR: 3.75), and frailty (aOR:3.03) significantly associated with this outcome (all p < 0.0001).
Conclusions: Dysgeusia appears predominant in MASLD-dACLD and, via appetite impairment, in a close relationship with malnutrition, sarcopenia, and frailty, negatively influencing patients' outcomes.
Keywords: Frailty; Liver cirrhosis; Nutrition; Sarcopenia; Translational Medicine.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study follows the Declaration of Helsinki (1975) and has been approved by the ethical committee of the University of Campania Luigi Vanvitelli in Naples (prot n. 0016948/i-2023). All study participants, or their legal guardians, provided informed written consent before study enrollment. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Red cell distribution width/platelet ratio estimates the 3-year risk of decompensation in Metabolic Dysfunction-Associated Steatotic Liver Disease-induced cirrhosis.World J Gastroenterol. 2024 Feb 21;30(7):685-704. doi: 10.3748/wjg.v30.i7.685. World J Gastroenterol. 2024. PMID: 38515952 Free PMC article.
-
Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality.J Hepatol. 2021 Apr;74(4):819-828. doi: 10.1016/j.jhep.2020.10.004. Epub 2020 Oct 16. J Hepatol. 2021. PMID: 33075344
-
Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis.Aliment Pharmacol Ther. 2020 Jan;51(1):64-77. doi: 10.1111/apt.15571. Epub 2019 Nov 8. Aliment Pharmacol Ther. 2020. PMID: 31701570 Review.
-
Chronic Liver Disease in the Older Patient-Evaluation and Management.Curr Gastroenterol Rep. 2023 Dec;25(12):390-400. doi: 10.1007/s11894-023-00908-2. Epub 2023 Nov 22. Curr Gastroenterol Rep. 2023. PMID: 37991713 Review.
-
A Comparative Study of Dietary Intake, Nutritional Status, and Frailty in Outpatients and Inpatients with Liver Cirrhosis.Nutrients. 2025 Feb 5;17(3):580. doi: 10.3390/nu17030580. Nutrients. 2025. PMID: 39940438 Free PMC article.
References
-
- Jafari A, Alaee A, Ghods K. The etiologies and considerations of dysgeusia: A review of literature. J Oral Biosci. 2021;63(4):319–26. - PubMed
-
- Bunchorntavakul C, Reddy KR. Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther. 2020;51(1):64–77. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

