Voltage-dependent anion channel 1 oligomerization regulates PANoptosis in retinal ischemia-reperfusion injury
- PMID: 39819824
- PMCID: PMC12407563
- DOI: 10.4103/NRR.NRR-D-24-00674
Voltage-dependent anion channel 1 oligomerization regulates PANoptosis in retinal ischemia-reperfusion injury
Abstract
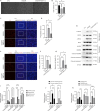
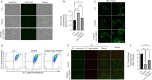
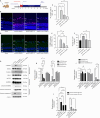
JOURNAL/nrgr/04.03/01300535-202604000-00045/figure1/v/2025-06-30T060627Z/r/image-tiff Ischemia-reperfusion injury is a common pathophysiological mechanism in retinal degeneration. PANoptosis is a newly defined integral form of regulated cell death that combines the key features of pyroptosis, apoptosis, and necroptosis. Oligomerization of mitochondrial voltage-dependent anion channel 1 is an important pathological event in regulating cell death in retinal ischemia-reperfusion injury. However, its role in PANoptosis remains largely unknown. In this study, we demonstrated that voltage-dependent anion channel 1 oligomerization-mediated mitochondrial dysfunction was associated with PANoptosis in retinal ischemia-reperfusion injury. Inhibition of voltage-dependent anion channel 1 oligomerization suppressed mitochondrial dysfunction and PANoptosis in retinal cells subjected to ischemia-reperfusion injury. Mechanistically, mitochondria-derived reactive oxygen species played a central role in the voltage-dependent anion channel 1-mediated regulation of PANoptosis by promoting PANoptosome assembly. Moreover, inhibiting voltage-dependent anion channel 1 oligomerization protected against PANoptosis in the retinas of rats subjected to ischemia-reperfusion injury. Overall, our findings reveal the critical role of voltage-dependent anion channel 1 oligomerization in regulating PANoptosis in retinal ischemia-reperfusion injury, highlighting voltage-dependent anion channel 1 as a promising therapeutic target.
Keywords: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PANoptosis; apoptosis; ischemia–reperfusion injury; mitochondrial dysfunction; necroptosis; oxidative stress; pyroptosis; reactive oxygen species; voltage-dependent anion channel 1.
Copyright © 2025 Neural Regeneration Research.
Conflict of interest statement
Figures





References
-
- Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100. - PubMed
-
- Chen H, Lu M, Lyu Q, Shi L, Zhou C, Li M, Feng S, Liang X, Zhou X, Ren L. Mitochondrial dynamics dysfunction: Unraveling the hidden link to depression. Biomed Pharmacother. 2024;175:116656. - PubMed
LinkOut - more resources
Full Text Sources

