Short- and long-term effects of transcutaneous spinal cord stimulation on autonomic cardiovascular control and arm-crank exercise capacity in individuals with a spinal cord injury (STIMEX-SCI): study protocol
- PMID: 39819908
- PMCID: PMC11751795
- DOI: 10.1136/bmjopen-2024-089756
Short- and long-term effects of transcutaneous spinal cord stimulation on autonomic cardiovascular control and arm-crank exercise capacity in individuals with a spinal cord injury (STIMEX-SCI): study protocol
Abstract
Introduction: Individuals with higher neurological levels of spinal cord injury (SCI) at or above the sixth thoracic segment (≥T6), exhibit impaired resting cardiovascular control and responses during upper-body exercise. Over time, impaired cardiovascular control predisposes individuals to lower cardiorespiratory fitness and thus a greater risk for cardiovascular disease and mortality. Non-invasive transcutaneous spinal cord stimulation (TSCS) has been shown to modulate cardiovascular responses at rest in individuals with SCI, yet its effectiveness to enhance exercise performance acutely, or promote superior physiological adaptations to exercise following an intervention, in an adequately powered cohort is unknown. Therefore, this study aims to explore the efficacy of acute TSCS for restoring autonomic function at rest and during arm-crank exercise to exhaustion (AIM 1) and investigate its longer-term impact on cardiorespiratory fitness and its concomitant benefits on cardiometabolic health and health-related quality of life (HRQoL) outcomes following an 8-week exercise intervention (AIM 2).
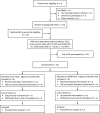
Methods and analysis: Sixteen individuals aged ≥16 years with a chronic, motor-complete SCI between the fifth cervical and sixth thoracic segments will undergo a baseline TSCS mapping session followed by an autonomic nervous system (ANS) stress test battery, with and without cardiovascular-optimised TSCS (CV-TSCS). Participants will then perform acute, single-session arm-crank exercise (ACE) trials to exhaustion with CV-TSCS or sham TSCS (SHAM-TSCS) in a randomised order. Twelve healthy, age- and sex-matched non-injured control participants will be recruited and will undergo the same ANS tests and exercise trials but without TSCS. Thereafter, the SCI cohort will be randomly assigned to an experimental (CV-TSCS+ACE) or control (SHAM-TSCS+ACE) group. All participants will perform 48 min of ACE twice per week (at workloads corresponding to 73-79% peak oxygen uptake), over a period of 8 weeks, either with (CV-TSCS) or without (SHAM-TSCS) cardiovascular-optimised stimulation. The primary outcomes are time to exhaustion (AIM 1) and cardiorespiratory fitness (AIM 2). Secondary outcomes for AIM 1 include arterial blood pressure, respiratory function, cerebral blood velocity, skeletal muscle tissue oxygenation, along with concentrations of catecholamines, brain-derived neurotrophic factor and immune cell dynamics via venous blood sampling pre, post and 90 min post-exercise. Secondary outcomes for AIM 2 include cardiometabolic health biomarkers, cardiac function, arterial stiffness, 24-hour blood pressure lability, energy expenditure, respiratory function, neural drive to respiratory muscles, seated balance and HRQoL (eg, bowel, bladder and sexual function). Outcome measures will be assessed at baseline, pre-intervention, post-intervention and after a 6-week follow-up period (HRQoL questionnaires only).
Ethics and dissemination: Ethical approval has been obtained from the Wales Research Ethics Committee 7 (23/WA/0284; 03/11/2024). The recruitment process began in February 2024, with the first enrolment in July 2024. Recruitment is expected to be completed by January 2026. The results will be presented at international SCI and sport-medicine conferences and will be submitted for publication in peer-reviewed journals.
Trial registration number: ISRCTN17856698.
Keywords: CARDIOLOGY; Neurological injury; Physical Therapy Modalities; REHABILITATION MEDICINE; SPORTS MEDICINE; Spine.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
