How many stroke survivors develop problematic spasticity requiring pharmacological therapy? An international (Europe and USA) observational study protocol
- PMID: 39819949
- PMCID: PMC11751951
- DOI: 10.1136/bmjopen-2024-087404
How many stroke survivors develop problematic spasticity requiring pharmacological therapy? An international (Europe and USA) observational study protocol
Abstract
Introduction: Current care plans for stroke survivors typically focus on acute management, resulting in many stroke survivors being discharged to their communities without adequate follow-up, despite their often experiencing significant post-stroke complications, such as post-stroke spasticity (PSS). While studies have explored the incidence and prevalence of PSS, little is known about how early PSS develops and how many stroke survivors develop 'problematic' PSS that would benefit from pharmacological treatment.
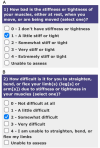
Methods and analysis: EPITOME is a prospective, international, observational, epidemiological study of participants (aged 18-90 years) who develop paresis within days 3-14 of a first-ever stroke that occurred within the past 4 weeks. Participants at sites across seven different countries are monitored remotely at 2 weeks and 1, 2, 3, 6, 9 and 12 months post-stroke to detect the possible onset of PSS using the Post-stroke Spasticity Monitoring Questionnaire (PSMQ). If the PSMQ indicates the possible presence of PSS, participants undergo a full in-clinic assessment to confirm the presence of PSS. For participants with confirmed PSS, the severity and distribution of spasticity is documented, and the investigator assesses whether the participant has spasticity that could benefit from pharmacological therapy. Participants without clinically confirmed PSS return to remote monitoring.
Ethics and dissemination: Ethics approval was obtained in all seven participating countries. Results will be published at international meetings and in an international peer-reviewed journal. Lay summaries will be prepared to accompany the primary paper and will also be provided to study participants.
Trial registration number: ClinicalTrials.gov NCT06055725.
Keywords: EPIDEMIOLOGIC STUDIES; Rehabilitation medicine; Stroke.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: RDZ, LSB, SB, SG, DHH, RP and AP are investigators in the EPITOME study and either they or their institutions receive payment for participation from Ipsen. In addition, RDZ reports research funding from Ipsen and AbbVie, consulting fees from Ipsen, royalties from Demos Medical for the Textbook of Stroke Recovery and Rehabilitation, and participation in a data safety monitoring board for SPR Therapeutics. SG reports research funding from Bundesministerium fur Bildung und Forschung (BMBF), Disabled Facilities Grants (DFG), Universitätsmedizin Mainz, Abbott, Boston Scientific, Böhringer Foundation, Magventure, Precisis, Innovationsfond GBA and lecture fees from Abbott, AbbVie, Bial, BVDN, Ipsen, and UCB. SS is a patient representative of Different Strokes and has no financial disclosures to report. SM, SP and PM are employed by Ipsen. AP reports consultancy for Ipsen, Merz and AbbVie.
Figures

References
-
- Feldman R, Young R, Koella W. Spasticity: disordered motor control. Chicago: Yearbook Medical Publishers; 1980. Pathophysiology of spasticity and clinical experience with baclofen; pp. 185–220.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
