Effectiveness of Buspirone in Alleviating Anxiety Symptoms in Patients with Depressive Disorder: A Multicenter Prospective Observational Study in Korea
- PMID: 39820120
- PMCID: PMC11747738
- DOI: 10.9758/cpn.24.1255
Effectiveness of Buspirone in Alleviating Anxiety Symptoms in Patients with Depressive Disorder: A Multicenter Prospective Observational Study in Korea
Abstract
Objective: We aimed to investigate the effectiveness of buspirone as an adjunctive therapy for alleviating anxiety symptoms in patients with depressive disorders who are already taking antidepressants.
Methods: This was an open-label prospective multicenter non-interventional observational study conducted over 12 weeks. We enrolled 180 patients diagnosed with depressive disorders according to DSM-5 criteria and Hamilton Anxiety Rating Scale (HAMA) scores ≥ 18. Participants were already taking selective serotonin reuptake inhibitors or serotoninnorepinephrine reuptake inhibitors and were prescribed adjunctive buspirone. Efficacy was assessed using HAMA, Hamilton Depression Rating Scale (HAMD), Clinical Global Impression Scale-Improvement, Clinical Global Impression Scale-Severity, Sheehan Disability Scale (SDS), and WHO-5 Well-Being Index.
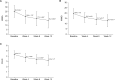
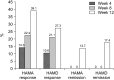
Results: The efficacy analysis included 161 patients. HAMA scores decreased significantly from 25.2 ± 6.7 at baseline to 15.4 ± 8.6 at 12 weeks (p < 0.001), whereas HAMD scores decreased from 19.4 ± 4.6 to 12.7 ± 5.7 (p < 0.001). WHO-5 and SDS scores showed significant improvements. The HAMA response rate was 39.1% and the remission rate was 13.7% at 12 weeks. Adverse drug reactions were reported in 3.7% of participants. Subgroup analyses showed no significant differences in treatment response based on buspirone dosage, baseline anxiety/depression severity, or benzodiazepine use.
Conclusion: Adjunctive buspirone therapy effectively improved anxiety symptoms in depressed patients taking antidepressants, regardless of baseline symptom severity or buspirone dosage. The treatment was well-tolerated with few adverse events. Future studies using a control group are needed.
Keywords: Anxiety; Anxious depression; Buspirone; Depression.
Conflict of interest statement
Won-Myong Bahk is a member of the Editorial Board of Clinical Psychopharmacology and Neuroscience, Chi-Un Pae is an editor-in-chief of Clinical Psychopharmacology and Neuroscience, Young-Eun Jung and Young Sup Woo are the associate editors of Clinical Psychopharmacology and Neuroscience. These authors were not involved in the journal’s review of, or decisions related to, this manuscript. No other potential conflicts of interest pertinent to this article were reported.
Figures

Similar articles
-
Effects of Jie Yu Wan on Generalized Anxiety Disorder: A Randomized Clinical Trial.Evid Based Complement Alternat Med. 2022 Apr 8;2022:9951693. doi: 10.1155/2022/9951693. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35432560 Free PMC article.
-
Duloxetine in the acute and long-term treatment of major depressive disorder: a placebo- and paroxetine-controlled trial.Eur Neuropsychopharmacol. 2004 Dec;14(6):457-70. doi: 10.1016/j.euroneuro.2004.01.002. Eur Neuropsychopharmacol. 2004. PMID: 15589385 Clinical Trial.
-
Efficacy, safety, and tolerability of venlafaxine extended release and buspirone in outpatients with generalized anxiety disorder.J Clin Psychiatry. 1999 Aug;60(8):528-35. doi: 10.4088/jcp.v60n0805. J Clin Psychiatry. 1999. PMID: 10485635 Clinical Trial.
-
Pharmacological interventions for treatment-resistant depression in adults.Cochrane Database Syst Rev. 2019 Dec 17;12(12):CD010557. doi: 10.1002/14651858.CD010557.pub2. Cochrane Database Syst Rev. 2019. PMID: 31846068 Free PMC article.
-
Buspirone in the long-term treatment of generalized anxiety disorder.J Clin Psychiatry. 1987 Dec;48 Suppl:3-6. J Clin Psychiatry. 1987. PMID: 3320034 Review.
References
-
- Shin C, Jeon SW, Lee SH, Pae CU, Hong N, Lim HK, et al. Efficacy and safety of escitalopram, desvenlafaxine, and vortioxetine in the acute treatment of anxious depression: a randomized rater-blinded 6-week clinical trial. Clin Psychopharmacol Neurosci. 2023;21:135–146. doi: 10.9758/cpn.2023.21.1.135. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

