Incomplete remyelination via therapeutically enhanced oligodendrogenesis is sufficient to recover visual cortical function
- PMID: 39820244
- PMCID: PMC11739692
- DOI: 10.1038/s41467-025-56092-6
Incomplete remyelination via therapeutically enhanced oligodendrogenesis is sufficient to recover visual cortical function
Abstract
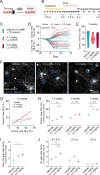
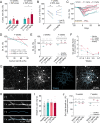
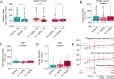
Myelin loss induces neural dysfunction and contributes to the pathophysiology of neurodegenerative diseases, injury conditions, and aging. Because remyelination is often incomplete, better understanding endogenous remyelination and developing remyelination therapies that restore neural function are clinical imperatives. Here, we use in vivo two-photon microscopy and electrophysiology to study the dynamics of endogenous and therapeutic-induced cortical remyelination and functional recovery after cuprizone-mediated demyelination in mice. We focus on the visual pathway, which is uniquely positioned to provide insights into structure-function relationships during de/remyelination. We show endogenous remyelination is driven by recent oligodendrocyte loss and is highly efficacious following mild demyelination, but fails to restore the oligodendrocyte population when high rates of oligodendrocyte loss occur quickly. Testing a thyromimetic (LL-341070) compared to clemastine, we find it better enhances oligodendrocyte gain and hastens recovery of neuronal function. The therapeutic benefit of the thyromimetic is temporally restricted, and it acts exclusively following moderate to severe demyelination, eliminating the endogenous remyelination deficit. However, we find regeneration of oligodendrocytes and myelin to healthy levels is not necessary for recovery of visual neuronal function. These findings advance our understanding of remyelination and its impact on functional recovery to inform future therapeutic strategies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: M.B.W., D.A.M., A.A., A.D., J.A.V., and R.G. were employees of Autobahn Therapeutics and hold equity interests in the company. J.A.V. remains an employee of Autobahn Therapeutics. Autobahn Therapeutics developed LL-341070 and provided LL-341070 for experiments. In vitro data and rat gene expression data (Supplementary Fig. 6) were provided by Autobahn Therapeutics. This project was funded in part by a Sponsored Research Agreement between Autobahn Therapeutics and E.G.H. and D.J.D.; academic freedom was maintained in designing and analyzing experiments and in writing the manuscript. All other authors have no other current or past financial involvements with Autobahn Therapeutics, or other competing interests to declare.
Figures




Update of
-
Incomplete remyelination via endogenous or therapeutically enhanced oligodendrogenesis is sufficient to recover visual cortical function.bioRxiv [Preprint]. 2024 Feb 22:2024.02.21.581491. doi: 10.1101/2024.02.21.581491. bioRxiv. 2024. Update in: Nat Commun. 2025 Jan 16;16(1):732. doi: 10.1038/s41467-025-56092-6. PMID: 39411163 Free PMC article. Updated. Preprint.
References
-
- Mcdonald, W. I. & Sears, T. A. Effect of Demyelination on Conduction in the Central Nervous System. Nature221, 182–183 (1969). - PubMed
-
- Smith, K. J., Blakemore, W. F. & Mcdonald, W. I. Central remyelination restores secure conduction. Nature280, 395–396 (1979). - PubMed
-
- Irvine, K. A. & Blakemore, W. F. Remyelination protects axons from demyelination-associated axon degeneration. Brain131, 1464–1477 (2008). - PubMed
MeSH terms
Substances
Grants and funding
- NS120850/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS134829/NS/NINDS NIH HHS/United States
- R01 NS125230/NS/NINDS NIH HHS/United States
- R01 NS120850/NS/NINDS NIH HHS/United States
- EY028612/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)
- HT9425-23-1-0561/U.S. Department of Defense (United States Department of Defense)
- FG-2107-38324/National Multiple Sclerosis Society (National MS Society)
- NS125230/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- NS115975/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R01 NS115975/NS/NINDS NIH HHS/United States
- K99 EY028612/EY/NEI NIH HHS/United States
- NS132859/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- NS134829/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R00 EY028612/EY/NEI NIH HHS/United States
- R01 NS132859/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources

