Melanophilin-induced primary cilia promote pancreatic cancer metastasis
- PMID: 39820281
- PMCID: PMC11739566
- DOI: 10.1038/s41419-025-07344-2
Melanophilin-induced primary cilia promote pancreatic cancer metastasis
Abstract
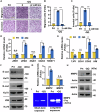
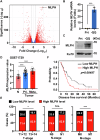
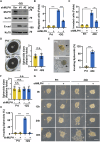
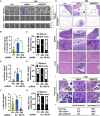
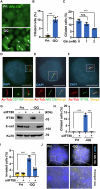
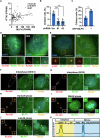
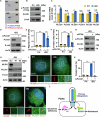
Pancreatic ductal adenocarcinoma (PDAC) is one of the most malignant tumors because of its high metastatic ability. The glutamine (Gln)-deficient microenvironment contributes to PDAC metastasis; however, the underlying molecular mechanisms remain unclear. Here, we demonstrated that melanophilin (MLPH) promotes PDAC metastasis by inducing the regrowth of primary cilia. Using RNA sequencing, we found that MLPH was upregulated in Gln-deficient conditions. MLPH facilitated PDAC metastasis in vitro and in vivo. Clinically, high MLPH expression is positively correlated with metastasis and poor PDAC prognosis. MLPH localized to the centrosome and facilitated the regrowth of primary cilia. The primary ciliogenesis upregulated phospholipase C γ-1 (PLCG1) to promote PDAC metastasis. Interestingly, PLCG1 was localized to the primary cilia, and depletion of PLCG1 alleviated primary ciliogenesis, suggesting a feedforward role for PLCG1 in mediating primary ciliogenesis. Thus, our study revealed a novel function of the MLPH-primary cilia-PLCG1 axis in facilitating PDAC metastasis under Gln deficiency both in vitro and in vivo.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval: All animal experiments were conducted in accordance with the guidelines approved by the animal ethics committee of National Cheng Kung University and complied with all relevant ethical guidelines (#113180). The retrospective clinical study was approved by the Institutional Review Board (IRB) of the NCKUH (IRB number: NCKUH B-ER-110-420) and was conducted following the ethical research guidelines.
Figures
Similar articles
-
HDAC2 promotes loss of primary cilia in pancreatic ductal adenocarcinoma.EMBO Rep. 2017 Feb;18(2):334-343. doi: 10.15252/embr.201541922. Epub 2016 Dec 27. EMBO Rep. 2017. PMID: 28028031 Free PMC article.
-
CALB2 drives pancreatic cancer metastasis through inflammatory reprogramming of the tumor microenvironment.J Exp Clin Cancer Res. 2024 Oct 3;43(1):277. doi: 10.1186/s13046-024-03201-w. J Exp Clin Cancer Res. 2024. PMID: 39358777 Free PMC article.
-
Ciliogenesis and Hedgehog signalling are suppressed downstream of KRAS during acinar-ductal metaplasia in mouse.Dis Model Mech. 2020 Jul 30;13(7):dmm044289. doi: 10.1242/dmm.044289. Dis Model Mech. 2020. PMID: 32571902 Free PMC article.
-
FBXO32 Stimulates Protein Synthesis to Drive Pancreatic Cancer Progression and Metastasis.Cancer Res. 2024 Aug 15;84(16):2607-2625. doi: 10.1158/0008-5472.CAN-23-3638. Cancer Res. 2024. PMID: 38775804
-
Macrophage-derived exosomal microRNA-501-3p promotes progression of pancreatic ductal adenocarcinoma through the TGFBR3-mediated TGF-β signaling pathway.J Exp Clin Cancer Res. 2019 Jul 15;38(1):310. doi: 10.1186/s13046-019-1313-x. J Exp Clin Cancer Res. 2019. PMID: 31307515 Free PMC article.
Cited by
-
Prostaglandin E2 and Progesterone Receptor Coordinately Regulate Primary Cilia for Proper Decidualization.FASEB J. 2025 Aug 15;39(15):e70919. doi: 10.1096/fj.202500961RR. FASEB J. 2025. PMID: 40772309 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

