Cigarette smoke components modulate the MR1-MAIT axis
- PMID: 39820322
- PMCID: PMC11740918
- DOI: 10.1084/jem.20240896
Cigarette smoke components modulate the MR1-MAIT axis
Abstract
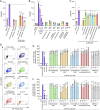
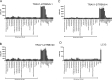
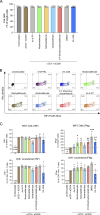
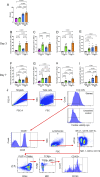
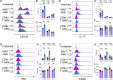
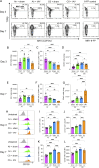
Tobacco smoking is prevalent across the world and causes numerous diseases. Cigarette smoke (CS) compromises immunity, yet little is known of the components of CS that impact T cell function. MR1 is a ubiquitous molecule that presents bacterial metabolites to MAIT cells, which are highly abundant in the lungs. Using in silico, cellular, and biochemical approaches, we identified components of CS that bind MR1 and impact MR1 cell surface expression. Compounds, including nicotinaldehyde, phenylpropanoid, and benzaldehyde-related scaffolds, bound within the A' pocket of MR1. CS inhibited MAIT cell activation, ex vivo, via TCR-dependent and TCR-independent mechanisms. Chronic CS exposure altered MAIT cell phenotype and function and attenuated MAIT cell responses to influenza A virus infection in vivo. MR1-deficient mice were partially protected from the development of chronic obstructive pulmonary disease (COPD) features that were associated with CS exposure. Thus, CS can impair MAIT cell function by diverse mechanisms, and potentially contribute to infection susceptibility and disease exacerbations.
© 2025 Awad et al.
Conflict of interest statement
Disclosures: L. Liu reported a patent to WO2015149130 licensed “National Institutes of Health (USA) and Immudex (Denmark)” and a patent to WO2014005194 licensed “National Institutes of Health (USA) and Immudex (Denmark).” J.Y.W. Mak reported a patent to WO2015149130A9 issued. J. McCluskey reported a patent to US 10011602B2, PCT/AU2013/000742 issued and a patent to US 10245262 B2 issued; and “MR1-Antigen dextramers are manufactured under a University of Melbourne license by Immudex MR1-Ag tetramers are manufactured and distributed by the NIH Tetramer facility at no cost to investigators.” A.J. Corbett reported a patent to WO2014/005194 and WO2015/149130 licensed “NIH Core tetramer facility” and a patent to WO2014/005194 and WO2015/149130 licensed “Immudex.” D.P. Fairlie reported a patent to WO/2015/149130 issued and a patent to WO/2014/005194 licensed “Immudex.” J. Rossjohn reported a patent to WO/2015/149130 issued and a patent to WO/2014/005194 licensed “Immudex.” No other disclosures were reported.
Figures








References
-
- Adams, P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.W., Kapral G.J., Grosse-Kunstleve R.W., et al. . 2010. PHENIX: A comprehensive python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66:213–221. 10.1107/S0907444909052925 - DOI - PMC - PubMed
-
- Beckett, E.L., Stevens R.L., Jarnicki A.G., Kim R.Y., Hanish I., Hansbro N.G., Deane A., Keely S., Horvat J.C., Yang M., et al. . 2013. A new short-term mouse model of chronic obstructive pulmonary disease identifies a role for mast cell tryptase in pathogenesis. J. Allergy Clin. Immunol. 131:752–762. 10.1016/j.jaci.2012.11.053 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

