Phase 1 study of quercetin, a natural antioxidant for children and young adults with Fanconi anemia
- PMID: 39820512
- PMCID: PMC12008688
- DOI: 10.1182/bloodadvances.2024015053
Phase 1 study of quercetin, a natural antioxidant for children and young adults with Fanconi anemia
Abstract
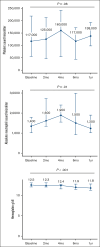
Fanconi anemia (FA) is a rare inherited disorder characterized by progressive bone marrow failure (BMF) and a predisposition to malignancy. Systemic reactive oxygen species (ROS) and increased sensitivity of FA hematopoietic progenitors to ROS play a key role in the pathogenesis of BMF. Treatment with antioxidants improve hematopoietic function in Fancc-/- mice. We report the safety, tolerability, and pharmacokinetics of quercetin, a naturally occurring antioxidant in the first dose-finding phase 1 study for patients with FA. Twelve patients (median age, 7 years [range, 3-21]) received oral quercetin twice daily for 4 months. Quercetin was well tolerated at all dose levels. Allometrically bodyweight-adjusted dose with a maximum adult daily dose of 4000 mg/d was established as the recommended dose of quercetin. Patients in an expansion cohort (n = 18) were treated using this recommended dose for 6 months. A subset of patients showed reduced ROS levels in the peripheral blood (PB) and bone marrow stem cell compartment. Patients in the analysis cohort treated with the recommended dose of quercetin achieved an a priori-defined optimal response of 25% reduction in the PB ROS level compared with baseline. Platelet counts remained stable to slightly improved over the study period (P = .06). Absolute neutrophil counts (P = .01) and hemoglobin levels gradually declined (P = .001). In those with evidence of BMF at baseline, 8 of 15 patients (53%) had a hematological response at some point after quercetin treatment. Fluctuations in counts are common in patients with FA, limiting accurate assessment of the impact of quercetin use in FA. This trial was registered at www.ClinicalTrials.gov as #NCT01720147.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: P.A.M. is a board member at Orthogon Therapeutics. D.E. receives research support from Dexcom and Sanofi. J.H. is a consultant for EMD Serono. K.C.M. receives research funding from Elixirgen Therapeutics and Incyte. K.D.R.S. holds equity in Asklepion Pharmaceuticals and Aliveris s.r.l.; and is a consultant for Mirum Pharmaceuticals. The remaining authors declare no competing financial interests.
Figures



References
-
- Kutler DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101(4):1249–1256. - PubMed
-
- Alter BP. Fanconi's anemia and malignancies. Am J Hematol. 1996;53(2):99–110. - PubMed
-
- Mehta PA, Ebens C. In: GeneReviews ® [Internet] Adam MP, Feldman J, Mirzaa GM, et al., editors. University of Washington, Seattle; Seattle (WA): 1993-2017. [updated March 2021]. Fanconi Anemia.
-
- Bogliolo M, Surralles J. Fanconi anemia: a model disease for studies on human genetics and advanced therapeutics. Curr Opin Genet Dev. 2015;33:32–40. - PubMed
-
- Rodriguez A, D'Andrea A. Fanconi anemia pathway. Curr Biol. 2017;27(18):R986–R988. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

