Once-weekly IcoSema versus once-weekly semaglutide in adults with type 2 diabetes: the COMBINE 2 randomised clinical trial
- PMID: 39820580
- PMCID: PMC11950020
- DOI: 10.1007/s00125-024-06348-5
Once-weekly IcoSema versus once-weekly semaglutide in adults with type 2 diabetes: the COMBINE 2 randomised clinical trial
Erratum in
-
Correction: Once-weekly IcoSema versus once-weekly semaglutide in adults with type 2 diabetes: the COMBINE 2 randomised clinical trial.Diabetologia. 2025 Jul;68(7):1586-1588. doi: 10.1007/s00125-025-06435-1. Diabetologia. 2025. PMID: 40323333 Free PMC article. No abstract available.
Abstract
Aims/hypothesis: COMBINE 2 assessed the efficacy and safety of once-weekly IcoSema (a combination therapy of basal insulin icodec and semaglutide) vs once-weekly semaglutide (a glucagon-like peptide-1 analogue) 1.0 mg in individuals with type 2 diabetes inadequately managed with GLP-1 receptor agonist (GLP-1 RA) therapy, with or without additional oral glucose-lowering medications.
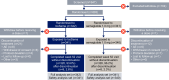
Methods: This 52 week, randomised, multicentre, open-label, parallel group, Phase IIIa trial was conducted across 121 sites in 13 countries/regions. Adults with type 2 diabetes (HbA1c 53.0-85.8 mmol/mol [7.0-10.0%]) receiving GLP-1 RA therapy with or without additional oral glucose-lowering medications were randomly assigned 1:1 to once-weekly IcoSema or once-weekly semaglutide 1.0 mg. The primary endpoint was change in HbA1c from baseline to week 52; superiority of IcoSema to semaglutide 1.0 mg was assessed. Secondary endpoints included change in fasting plasma glucose and body weight (baseline to week 52), and combined clinically significant (level 2; <3.0 mmol/l) or severe (level 3; associated with severe cognitive impairment requiring external assistance for recovery) hypoglycaemia (baseline to week 57).
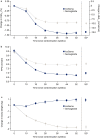
Results: Overall, 683 participants were randomised using a Randomisation and Trial Supply Management system to IcoSema (n=342) or semaglutide 1.0 mg (n=341). Mean ± SD baseline characteristics were as follows: HbA1c 64.0±8.2 mmol/mol (8.0±0.7%); diabetes duration 12.6±6.9 years; and BMI 31.1±4.7 kg/m2. From baseline to week 52, mean change in HbA1c was -14.7 mmol/mol (-1.35%-points) in the IcoSema group and -9.88 mmol/mol (-0.90%-points) in the semaglutide group; the estimated treatment difference (ETD) was -4.85 (95% CI -6.13, -3.57) mmol/mol (-0.44 [95% CI -0.56, -0.33]%-points), confirming superiority of IcoSema to semaglutide (p<0.0001). The estimated mean change in fasting plasma glucose from baseline to week 52 was statistically significantly reduced with IcoSema vs semaglutide (-2.48 mmol/l vs -1.43 mmol/l, respectively; ETD -1.05 [95% CI -1.36, -0.75] mmol; p<0.0001). Mean change in body weight from baseline to week 52 was statistically significantly different between groups: +0.84 kg for IcoSema vs -3.70 kg for semaglutide (ETD 4.54 kg [95% CI 3.84, 5.23]; p<0.0001). There was no statistically significant difference in the rate of combined clinically significant or severe hypoglycaemia between IcoSema and semaglutide (0.042 vs 0.036 episodes per person-year of exposure; estimated rate ratio 1.20 [95% CI 0.53, 2.69]; p=0.66). The proportion of participants experiencing gastrointestinal adverse events was similar between treatment groups (IcoSema 31.4%; semaglutide 34.4%).
Conclusions/interpretation: In people living with type 2 diabetes inadequately managed with GLP-1 RA therapy, with or without additional oral glucose-lowering medications, switching to once-weekly IcoSema in comparison with once-weekly semaglutide 1.0 mg demonstrated superiority in HbA1c reduction, similar rates of clinically significant or severe hypoglycaemia, and similar frequency of gastrointestinal adverse events. However, weight change from baseline to week 52 was statistically significantly in favour of semaglutide 1.0 mg.
Trial registration: ClinicalTrials.gov NCT05259033 FUNDING: This trial was funded by Novo Nordisk.
Keywords: Fixed-ratio combination; GLP-1 RA; Glycaemic control; Hypoglycaemia; IcoSema; Insulin icodec; Once-weekly; Safety; Semaglutide; Type 2 diabetes.
© 2025. The Author(s).
Conflict of interest statement
Acknowledgements: The authors thank all trial participants and staff. Medical writing support was provided by G. Rogers and J. Geatrell of Oxford PharmaGenesis, Oxford, UK, funded by Novo Nordisk A/S. Data availability: Individual participant data will be shared in data sets in a de-identified/anonymised format. Shared data will include data sets from Novo Nordisk-sponsored clinical research completed after 2001 for product indications approved in both the EU and the USA. The trial protocol and redacted clinical trial report will be made available according to Novo Nordisk data sharing commitments. These data will be available after research completion and approval of product and product use in both the EU and the USA (no end date). Data will be shared with bona fide researchers submitting a research proposal requesting access to data, for use as approved by the Independent Review Board (IRB) according to the IRB charter (see novonordisk-trials.com). These data can be accessed via an access request proposal form; the access criteria can be found at novonordisk-trials.com. The data will be made available on a specialised SAS data platform. The results tables will be made available according to US and EU law, via Clinicaltrials.gov and EU Clinical Trials Register. Clinical trials synopsis will be uploaded to novonordisk-trials.com for clinical projects that have been discontinued. Funding: This trial was funded by Novo Nordisk A/S. Representatives of Novo Nordisk A/S were involved in the design and conduct of the trial; collection, management, analysis and interpretation of the data; and preparation, review and approval of the manuscript. Investigators were responsible for trial-related medical decisions and data collection; Novo Nordisk undertook site monitoring, data collation and analysis. Medical writers (G. Rogers and J. Geatrell from Oxford PharmaGenesis, Oxford, UK), funded by Novo Nordisk, assisted with drafting the manuscript under direction of the authors. Novo Nordisk did not have the right to veto publication or to control the decision regarding the journal to which the manuscript was submitted; these decisions were made by the authors. Authors’ relationships and activities: IL has received research funding (paid to the institution they are affiliated with) from Boehringer Ingelheim, Merck, Mylan, Novo Nordisk, Pfizer and Sanofi, and has received advisory/consulting fees and/or other support from AstraZeneca, Bayer, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, GI Dynamics, Intarcia Therapeutics, Intercept Pharmaceuticals, Johnson & Johnson, MannKind, Merck, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi, Shionogi, Structure Therapeutics, Target Pharma, Valeritas and Zealand Pharma. MB and AF are employees of Novo Nordisk A/S. LC has received research funding (paid to the institution they are affiliated with) from AstraZeneca and Eli Lilly. EJ has received research funding (paid to the institution they are affiliated with) from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Faes Farma, MSD, Novartis and Novo Nordisk, and has received advisory/consulting fees and/or other support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, MSD, Novo Nordisk, Technofarma, UCB and Viatris. TN is an employee of Novo Nordisk Pharma Ltd. J-PR is an advisory panel member for Abbott, Air Liquide International, Alphadiab, AstraZeneca, Dexcom, Eli Lilly, Medtronic, MSD, Novo Nordisk and Sanofi, and has received research funding from and provided research support to Abbott, Air Liquide International, Medtronic, Novo Nordisk and Sanofi. DY has received consulting or speaker fees from Eli Lilly Japan K.K., Mitsubishi Tanabe Pharma, MSD K.K., Nippon Boehringer Ingelheim, Novo Nordisk Pharma Ltd and Sumitomo Pharma, and clinically commissioned/received joint research grants from Arklay, Nippon Boehringer Ingelheim, Novo Nordisk Pharma Ltd and Taisho Pharmaceutical. TZ has received research funding (paid to the institution they are affiliated with) from Akero Therapeutics, Novartis and Novo Nordisk, and has received advisory/consulting fees and/or other support (paid to the institution they are affiliated with) from AstraZeneca, Eli Lilly, Novo Nordisk, and Sanofi. RR is an advisory panel member for AstraZeneca, Boehringer Ingelheim, Eli Lilly and Novo Nordisk, has participated in speakers’ bureaus for AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD and Novo Nordisk, and has received research grants from Boehringer Ingelheim, Eli Lilly and Novo Nordisk. Contribution statement: All authors had full access to the data associated with this trial and contributed to interpreting the data and drafting the manuscript. The authors jointly decided to submit the manuscript, approved it before submission and take full responsibility for its content. IL is the guarantor of this work.
Figures
References
-
- Philis-Tsimikas A, Asong M, Franek E et al (2023) Switching to once-weekly insulin icodec versus once-daily insulin degludec in individuals with basal insulin-treated type 2 diabetes (ONWARDS 2): a phase 3a, randomised, open label, multicentre, treat-to-target trial. Lancet Diabetes Endocrinol 11(6):414–425. 10.1016/S2213-8587(23)00093-1 - PubMed
-
- Rosenstock J, Bain SC, Gowda A et al (2023) Weekly Icodec versus daily glargine U100 in type 2 diabetes without previous insulin. N Engl J Med 389(4):297–308. 10.1056/NEJMoa2303208 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

