TP53 mutations are associated with CD19- relapse and inferior outcomes after blinatumomab in adults with ALL
- PMID: 39820649
- PMCID: PMC12242962
- DOI: 10.1182/bloodadvances.2024014986
TP53 mutations are associated with CD19- relapse and inferior outcomes after blinatumomab in adults with ALL
Abstract
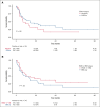
Despite the success of the CD19 × CD3 T-cell engager blinatumomab in B-cell acute lymphoblastic leukemia (B-ALL), treatment failure is common and can manifest with antigen loss and extramedullary disease (EMD) relapse. To understand the impact of leukemia genetics on outcomes, we reviewed 267 adult patients with B-ALL treated with blinatumomab and used next-generation sequencing to identify molecular alterations. Patients received blinatumomab for relapsed/refractory (R/R) disease (n = 150) and minimal residual disease (MRD; n = 88), upfront as induction (n = 10) or as consolidation in MRD-negative state (n = 19). In the overall cohort, 50 patients (19%) had Philadelphia chromosome (Ph)-positive ALL, 80 (30%) had Ph-like ALL, 35 (13%) had TP53 mutations (TP53m), 7 (3%) had KMT2A rearrangement, and 8 (3%) had PAX5 alterations. For patients treated for R/R ALL, the overall complete remission (CR)/CR with incomplete hematological recovery (CRi) rate was 59%. Only pretreatment high disease burden (P < .01) and active EMD (P < .01) were associated with inferior CR/CRi rate. Of 169 patients in CR/CRi after blinatumomab, 79 (47%) patients relapsed, including 22 (28%) with CD19- and 54 (68%) with CD19+ relapse. In multivariable analysis, TP53m was associated with an increased risk of CD19- relapse (hazard ratio [HR], 6.84; 95% confidence interval [CI], 2.68-17.45; P < .01). Post-blinatumomab allogeneic stem cell transplantation consolidation was associated with a lower risk of CD19- relapse (HR, 0.10; 95% CI, 0.03-0.37; P < .01) and EMD relapse (HR, 0.36; 95% CI, 0.18-0.73; P < .01). In conclusion, leukemia genetics may predict patterns of blinatumomab failure, with TP53m associated with CD19- relapse.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: I. Aldoss reports advisory board fees from Amgen, Kite, Pfizer, Jazz, AbbVie, Adaptive, Takeda, Syndax, and Agios; consulting fees from Pfizer, Takeda, Jazz, and Amgen; and research support from AbbVie, MacroGenics, and Jazz. M.M.A.M. reports advisory board fees from Hansa Biopharma, Stemline Therapeutics, Gilead, and Incyte; consultant fees from NexImmune, TScan, Tr1X, and CareDx; and research funding from NexImmune, Gilead, Miltenyi Biotec, and Incyte. L.M. reports advisory board fees from Jazz. A. Stein is a member of the speakers’ bureau for Amgen. G.M. is a member of the speakers’ bureau for AbbVie. V.P. has served on the advisory boards for AbbVie and Jazz Pharmaceuticals, and is a member of the speakers’ bureaus for Jazz Pharmaceuticals, Amgen, Novartis, and AbbVie. P.K. reports advisory board/consulting fees from Ascentage, Daiichi Sankyo, Bristol Myers Squibb, Novartis, and Takeda, and has served on the safety review committee with Treadwell Therapeutics. P.S.B. reports institutional research funding from GPCR Therapeutics; has served on the scientific advisory board for Patient Advocate Foundation; and has served on the medical advisory board for the AAMDS International Foundation. The remaining authors declare no competing financial interests.
Figures




References
-
- Aldoss I, Shah BD, Park JH, et al. Sequencing antigen-targeting antibodies and cellular therapies in adults with relapsed/refractory B-cell acute lymphoblastic leukemia. Am J Hematol. 2023;98(4):666–680. - PubMed
-
- Stelljes M, Raffel S, Alakel N, et al. Inotuzumab ozogamicin as induction therapy for patients older than 55 years with Philadelphia chromosome-negative B-precursor ALL. J Clin Oncol. 2024;42(3):273–282. - PubMed
-
- Topp MS, Gokbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57–66. - PubMed
-
- Topp MS, Gokbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–4140. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

