A human oral commensal-mediated protection against Sjögren's syndrome with maintenance of T cell immune homeostasis and improved oral microbiota
- PMID: 39820778
- PMCID: PMC11739518
- DOI: 10.1038/s41522-025-00654-5
A human oral commensal-mediated protection against Sjögren's syndrome with maintenance of T cell immune homeostasis and improved oral microbiota
Abstract
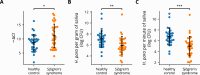
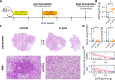
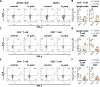
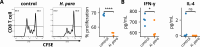
Sjögren's syndrome (SS) is a prevalent systemic autoimmune disease with substantial impacts on women's health worldwide. Although oral Haemophilus parainfluenzae is reduced in SS, its significance remains unclear. This study aimed to elucidate the pathophysiological role of H. parainfluenzae in SS. Reduced salivary H. parainfluenzae levels in SS patients were confirmed through quantitative PCR. Oral H. parainfluenzae inoculation in NOD mice alleviated focal sialadenitis, improved salivary function, and reduced IFN-γ+CD3+ and IFN-γ+CD8+ T cells in salivary gland-draining lymph nodes, maintaining immune homeostasis against a biased type 1 response. Inoculation also enhanced salivary microbiota diversity, balanced the Firmicutes-to-Proteobacteria ratio, and reduced the overwhelming presence of Pseudomonas mendocina. In vitro, H. parainfluenzae-preconditioned A253 cells limited CD8 T cell expansion with reduced IFN-γ production. These findings suggest that H. parainfluenzae improves oral microbial diversity, promotes homeostatic T-cell immunity, and protects against SS, supporting its potential as a next-generation probiotic.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Ramos-Casals, M. et al. Primary Sjögren syndrome in Spain: clinical and immunologic expression in 1010 patients. Medicine87, 210–219 (2008). - PubMed
-
- Weng, M.-Y., Huang, Y.-T., Liu, M.-F. & Lu, T.-H. Incidence of cancer in a nationwide population cohort of 7852 patients with primary Sjogren’s syndrome in Taiwan. Ann. Rheum. Dis.71, 524–527 (2012). - PubMed
-
- Meijer, J. M. et al. Health-related quality of life, employment and disability in patients with Sjogren’s syndrome. Rheumatol. Oxf. Engl.48, 1077–1082 (2009). - PubMed
-
- Qin, B. et al. Epidemiology of primary Sjögren’s syndrome: a systematic review and meta-analysis. Ann. Rheum. Dis.74, 1983–1989 (2015). - PubMed
-
- Brito-Zerón, P. et al. Sjögren syndrome. Nat. Rev. Dis. Prim.2, 16047 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

