Competitive signaling and cellular communications in myocardial infarction response
- PMID: 39820809
- PMCID: PMC11739196
- DOI: 10.1007/s11033-025-10236-5
Competitive signaling and cellular communications in myocardial infarction response
Abstract
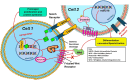
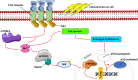
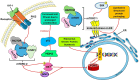
Cell communication and competition pathways are malleable to Myocardial Infarction (MI). Key signals, transcriptive regulators, and metabolites associated with apoptotic responses such as Myc, mTOR, and p53 are important players in the myocardium. The individual state of cardiomyocytes, fibroblasts, and macrophages in the heart tissue are adaptable in times of stress. The overlapping communication pathways of Wnt/β-catenin, Notch, and c-Kit exhibit the involvement of important factors in cell competition in the myocardium. Depending on the effects of these pathways on genetic expression and signal amplification, the proliferative capacities of the previously stated cells that make up the myocardium, amplify or diminish. This creates a distinct classification of "fit" and "unfit" cells. Beyond straightforward traits, the intricate metabolite interactions between neighboring cells unveil a complex battle. Strategic manipulation of these pathways holds translational promise for rapid cardiac recovery post-trauma.
Keywords: Cardiac healing; Cardiac regeneration; Cell communication; Cell competition; Myocardial infarction.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Exosomes Induce Crosstalk Between Multiple Types of Cells and Cardiac Fibroblasts: Therapeutic Potential for Remodeling After Myocardial Infarction.Int J Nanomedicine. 2024 Oct 19;19:10605-10621. doi: 10.2147/IJN.S476995. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39445157 Free PMC article. Review.
-
Regenerative cross talk between cardiac cells and macrophages.Am J Physiol Heart Circ Physiol. 2021 Jun 1;320(6):H2211-H2221. doi: 10.1152/ajpheart.00056.2021. Epub 2021 Mar 26. Am J Physiol Heart Circ Physiol. 2021. PMID: 33769920 Free PMC article.
-
Beta-catenin overexpression reduces myocardial infarct size through differential effects on cardiomyocytes and cardiac fibroblasts.J Biol Chem. 2006 Oct 13;281(41):30979-89. doi: 10.1074/jbc.M603916200. Epub 2006 Aug 18. J Biol Chem. 2006. PMID: 16920707
-
Stem cell factor gene transfer promotes cardiac repair after myocardial infarction via in situ recruitment and expansion of c-kit+ cells.Circ Res. 2012 Nov 9;111(11):1434-45. doi: 10.1161/CIRCRESAHA.111.263830. Epub 2012 Aug 29. Circ Res. 2012. PMID: 22931954 Free PMC article.
-
Recent Insights into Endogenous Mammalian Cardiac Regeneration Post-Myocardial Infarction.Int J Mol Sci. 2024 Nov 1;25(21):11747. doi: 10.3390/ijms252111747. Int J Mol Sci. 2024. PMID: 39519298 Free PMC article. Review.
References
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS (2019) S.S. Virani, null null, Heart Disease and Stroke Statistics—2019 Update: A Report From the American Heart Association, Circulation 139 e56–e528. 10.1161/CIR.0000000000000659 - PubMed
-
- Bayes-Genis A, Roura S, Prat-Vidal C, Farré J, Soler-Botija C, de Luna AB, Cinca J (2007) Chimerism and microchimerism of the human heart: evidence for cardiac regeneration. Nat Rev Cardiol 4:S40–S45. 10.1038/ncpcardio0748 - PubMed
-
- Lima A, Lubatti G, Burgstaller J, Hu D, Green AP, Di Gregorio A, Zawadzki T, Pernaute B, Mahammadov E, Perez-Montero S, Dore M, Sanchez JM, Bowling S, Sancho M, Kolbe T, Karimi MM, Carling D, Jones N, Srinivas S, Scialdone A, Rodriguez TA (2021) Cell competition acts as a purifying selection to eliminate cells with mitochondrial defects during early mouse development. Nat Metab 3:1091–1108. 10.1038/s42255-021-00422-7 - PMC - PubMed
-
- Gotthardt M, Raddatz K (2006) Cardiac Signaling: Cellular, Molecular and clinical aspects. Encyclopedic reference of Genomics and Proteomics in Molecular Medicine. Springer, Berlin, Heidelberg, pp 208–214. 10.1007/3-540-29623-9_4440.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

