Tofersen for SOD1 amyotrophic lateral sclerosis: a systematic review and meta-analysis
- PMID: 39820998
- PMCID: PMC12003547
- DOI: 10.1007/s10072-025-07994-2
Tofersen for SOD1 amyotrophic lateral sclerosis: a systematic review and meta-analysis
Abstract
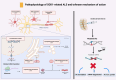
Objective: Tofersen, an antisense oligonucleotide, has recently received FDA and EMA approval for treating amyotrophic lateral sclerosis (ALS) in adults with SOD1 gene mutations. This systematic review and meta-analysis synthesized evidence on tofersen's safety and efficacy in patients with SOD1-related ALS.
Methods: A comprehensive search of three databases was conducted from inception through October 2024. Eligible studies included clinical trials, observational studies, and case studies. Meta-analyses were conducted using a random-effects model in RevMan.
Results: Twelve studies involving 195 patients treated with tofersen met the inclusion criteria, comprising two randomized controlled trials (RCTs), five cohort studies, one case series, and four case reports. Tofersen demonstrated promising effects, notably reducing SOD1 levels in cerebrospinal fluid and neurofilament light chain (NfL) in plasma, a biomarker strongly correlated with ALS progression and survival. Meta-analysis of RCTs showed a significantly lower rate of decline in ALS Functional Rating Scale-Revised (ALSFRS-R) scores from baseline in the tofersen group compared to placebo (SMD = 0.44, 95% CI [0.05 to 0.83], P = 0.03) and a significant reduction in the decline of predicted Slow Vital Capacity (P = 0.005). In a pre-post meta-analysis of five studies, a significant decrease in ALS progression rate (ALSFRS-R decline rate) was observed (MD = -0.28, 95% CI [-0.40 to -0.15], P < 0.0001). Reported adverse events were consistent with ALS progression or procedural effects.
Conclusion: Current evidence suggests that tofersen effectively reduces SOD1 and NfL levels and slow disease progression in SOD1 ALS, showing promise as a targeted therapeutic option.
Keywords: SOD1; ALS; Amyotrophic lateral sclerosis; Antisense oligonucleotide; Tofersen.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Not applicable. Consent for publication: Not applicable. Conflict of interests: The authors have no relevant financial or non-financial interests to disclose.
Figures



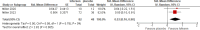
References
-
- Zou Z-Y, Zhou Z-R, Che C-H et al (2017) Genetic epidemiology of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 88. 10.1136/jnnp-2016-315018 - PubMed
-
- Hardiman O, Al-Chalabi A, Chio A et al (2017) Amyotrophic lateral sclerosis. Nat Rev Dis Prim 3:17071. 10.1038/nrdp.2017.71 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

